Author: Kelsey Zhao | Reviewers: GF Strong OTs and PTs | Published: 11 June 2024 | Updated: ~
For more information on abdominal binders, visit: community.scireproject.com/topic/abdominal-binders/
Author: Kelsey Zhao | Reviewers: GF Strong OTs and PTs | Published: 11 June 2024 | Updated: ~
For more information on abdominal binders, visit: community.scireproject.com/topic/abdominal-binders/
Author: Sharon Jang | Reviewer: Cathy Nevens | Published: 7 September 2019 | Updated: ~
Abdominal binders are simple pieces of equipment that are used to support the abdomen. This page outlines what abdominal binders are and how they are used after spinal cord injury (SCI).
Key Points

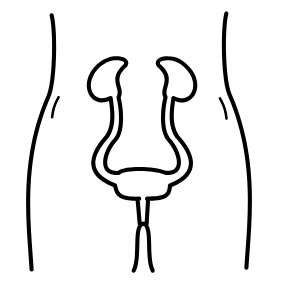
Abdominal binders wrap around to support the abdomen when the abdominal muscles are weak or paralyzed. They are normally worn under the shirt.1
An abdominal binder is an elastic piece of material that is placed normally placed around the lower torso to apply pressure to the abdomen. Abdominal binders should fit snugly around the torso and be tight enough to provide support, but should not be uncomfortable. Abdominal binders are typically worn under the shirt and are mainly used to improve circulation and breathing when in an upright position. They are also sometimes used to help maintain balance and stability of the trunk and to support sagging of the abdomen (sometimes called “quad belly”) that can happen when the abdominal muscles are weak.
There are two main types of abdominal binders: elastic and non-elastic binders. Each of these types will have a number of different models or designs available that may be used. Talk to your health providers about what type are most appropriate for you.
The most common type of abdominal binder used by people with SCI is made from a stretchy elastic fabric that is placed around the abdomen and closed with Velcro. The material mimics the nature of the abdominal muscles by providing some pressure but also allowing the abdomen to expand and recoil while breathing. Some elastic abdominal binders have additional supports built into them that may be used to assist with balance and stability.
Non-elastic abdominal binders include pieces of equipment such as a corset, girdle, straps or mechanical device to support the abdomen. These are made from a non-stretchy material that provides greater support. Non-elastic abdominal binders are not used as often after SCI because they have greater potential to injure the skin and may also restrict the abdomen while breathing, which may contribute to an abnormal breathing pattern.
 People with cervical and thoracic SCI may experience breathing problems because of a loss of nerve control to the diaphragm and other breathing muscles (including the abdominal muscles). This causes the diaphragm to sit too low in the abdomen so it cannot work optimally.
People with cervical and thoracic SCI may experience breathing problems because of a loss of nerve control to the diaphragm and other breathing muscles (including the abdominal muscles). This causes the diaphragm to sit too low in the abdomen so it cannot work optimally.
Abdominal binders are thought to mimic some of the function of the abdominal muscles to help support breathing. The binder compresses the abdomen, which increases pressure and may help to raise the diaphragm into a better position for breathing.
There is evidence that abdominal binding in people with tetraplegia can improve respiratory function. Studies have shown that the use of abdominal binders can improve an individual’s ability to inhale and exhale. Overtime, the use of an abdominal binder can strengthen the muscles that are used to inhale. The design of the abdominal binder may also influence its effectiveness. For example, one (weak evidence) study found that a custom girdle may cause individuals to perceive breathing as easier.
More research is required to find out how using an abdominal binder strengthens the diaphragm and whether this leads to easier breathing. Abdominal binding for people with SCI should be introduced gradually due to potential adverse effects on one’s ability to breathe.
Refer to our article on Respiratory Changes After SCI for more information!
 Many people with SCI experience a drop in blood pressure when moving from a lying or sitting position to an upright position. This is known as orthostatic hypotension. This condition happens because a loss of nerve function can impair the body’s ability to tighten (constrict) the blood vessels and change heart rate, which is an important part of maintaining blood pressure in different positions.
Many people with SCI experience a drop in blood pressure when moving from a lying or sitting position to an upright position. This is known as orthostatic hypotension. This condition happens because a loss of nerve function can impair the body’s ability to tighten (constrict) the blood vessels and change heart rate, which is an important part of maintaining blood pressure in different positions.
Because abdominal binders wrap around and compress the abdomen, they may help to increase pressure in the abdominal area. This may help to prevent blood pooling in the blood vessels in the abdomen when upright, which may help maintain blood pressure and allow better circulation.
There is conflicting evidence based on limited research that abdominal binders have any effect on cardiovascular responses in people with SCI. One study found that abdominal binders do not have any effect on average blood pressure or other cardiovascular responses. However, other studies suggest that abdominal binders in combination with leg stockings may have an effect on cardiovascular responses during lower intensity arm exercise.
Refer to our article on Orthostatic Hypotension for more information!
 There is some weak evidence for the use of an abdominal binder to improve speech. One study found that participants with difficulty speaking due to cervical level injuries were able to produce more natural sounding speech, were able to speak louder, and improved overall voice quality with the use of an abdominal binder. Meanwhile, another study has shown that using an abdominal binder can extend the length that sound is produced for.
There is some weak evidence for the use of an abdominal binder to improve speech. One study found that participants with difficulty speaking due to cervical level injuries were able to produce more natural sounding speech, were able to speak louder, and improved overall voice quality with the use of an abdominal binder. Meanwhile, another study has shown that using an abdominal binder can extend the length that sound is produced for.
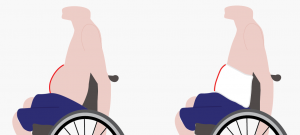
Abdominal binders may be used to support the abdomen to reduce the appearance of “quad belly”.5
Some individuals use an abdominal binder as they perceive it helps to support their trunk or assist with sitting balance. In addition, some individuals find that it helps them balance when performing two-handed exercises. However, no research has been done to support this. Some individuals may use a binder to reduce the appearance of the abdomen sagging forward, sometimes called “quad belly”.
Abdominal binders are considered safe for most people. In all cases, the skin under the binder should be regularly checked for pressure sores. However, there are situations in which abdominal binders may not be appropriate and carry possible risks. Please consult a health provider for detailed safety information.
Refer to our articles on Pressure Injuries, Spasticity, and Autonomic Dysreflexia for more information!
Abdominal binders are a physical treatment that supports the abdomen when the abdominal muscles are weak or paralyzed.
The support from the binder can improve cardiovascular and respiratory responses including blood pressure and breathing.
Some individuals may use a binder to help with balance and stabilizing the trunk but there is no research evidence to support this.
Some individuals may use a binder to reduce the appearance of abdominal muscles that bulge out following paralysis.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
This page has been adapted from SCIRE Professional “Respiratory Management” and “Orthostatic Hypotension” Modules:
Sheel AW, Reid WD, Townson AF (2018). Respiratory Management Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, Sproule S, Querée M, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0. Vancouver: p. 1-72.
Available from: scireproject.com/evidence/respiratory-management/
Krassioukov A, Wecht JM, Teasell RW, Eng JJ (2014). Orthostatic Hypotension Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 26.
Available from: scireproject.com/evidence/orthostatic-hypotension/non-pharmacological-management-of-oh/
Cornwell, P., Ward, E., Lim, Y., & Wadsworth, B. (2014). Impact of an Abdominal Binder on Speech Outcomes in People With Tetraplegic Spinal Cord Injury: Perceptual and Acoustic Measures. Topics in Spinal Cord Injury Rehabilitation, 20(1), 48–57. https://doi.org/10.1310/sci2001-48
Wadsworth, B. M., Haines, T. P., Cornwell, P. L., Rodwell, L. T., & Paratz, J. D. (2012). Abdominal Binder Improves Lung Volumes and Voice in People With Tetraplegic Spinal Cord Injury. YAPMR, 93(12), 2189–2197. https://doi.org/10.1016/j.apmr.2012.06.010

Author: Dominik Zbogar | Reviewer: Kathleen Martin Ginis | Published: 25 March 2019 | Updated: 30 June 2023
Physical activity is an important consideration after spinal cord injury (SCI) and is a key factor in preventing lifestyle diseases. This page provides guidelines for physical activity following SCI.
Key Points
 A group led by Dr. Kathleen Martin Ginis at the University of British Columbia and Dr. Victoria Goosey-Tolfrey at Loughborough University, UK developed international guidelines on exercise after SCI. The process of developing these guidelines involved a systematic review of relevant literature, consensus meetings, stakeholder feedback, and a formal audit of the process.
A group led by Dr. Kathleen Martin Ginis at the University of British Columbia and Dr. Victoria Goosey-Tolfrey at Loughborough University, UK developed international guidelines on exercise after SCI. The process of developing these guidelines involved a systematic review of relevant literature, consensus meetings, stakeholder feedback, and a formal audit of the process.
The result was the publication of the journal article about the guidelines (available in the journal Spinal Cord via nature.com/articles/s41393-017-0017-3) and the actual guidelines titled, “Scientific Exercise Guidelines for Adults with Spinal Cord Injury” which are described next.

Cardiometabolic health encompasses measures of body composition (e.g., fat mass, lean body mass) and risk factors for cardiovascular disease (e.g., high cholesterol and hypertension). Common measures to assess cardiometabolic health include a blood test to assess triglycerides and cholesterol, measuring blood pressure, and measuring height, weight, and waist circumference.
Cardiovascular fitness refers to the ability of the heart and lungs to deliver oxygen to working muscles and can be assessed through a maximal graded exercise test which provides information such as peak oxygen uptake and peak power output.
Muscle strength refers to the amount of force that a muscle can exert. It can be measured by lifting objects of specified weight or exerting force against a measurement tool such as a hand grip dynanometer.
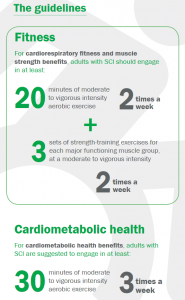
These exercise guidelines provide minimum thresholds for improving cardiorespiratory fitness and muscle strength and for improving cardiometabolic health.
 If you are not already exercising, it is okay to start with smaller amounts of exercise and gradually increase duration, frequency, and intensity, as a progression toward meeting the guidelines. Doing exercise below the recommended levels may or may not bring small changes in fitness or cardiometabolic health.
If you are not already exercising, it is okay to start with smaller amounts of exercise and gradually increase duration, frequency, and intensity, as a progression toward meeting the guidelines. Doing exercise below the recommended levels may or may not bring small changes in fitness or cardiometabolic health.
 Exceeding these exercise guidelines would be expected to yield additional cardiorespiratory fitness and muscle strength and cardiometabolic health benefits. However, there are insufficient data to comment on the risks associated with a person with SCI exceeding these guidelines.
Exceeding these exercise guidelines would be expected to yield additional cardiorespiratory fitness and muscle strength and cardiometabolic health benefits. However, there are insufficient data to comment on the risks associated with a person with SCI exceeding these guidelines.
The guidelines should be achieved above and beyond the incidental physical activity one might accumulate in the course of daily living. Adults are encouraged to participate routinely in exercise modalities and contexts that are sustainable, enjoyable, safe and reasonably achievable.
Refer to our article on Physical Activity for more information!
These guidelines are appropriate for adults (aged 18-64) with chronic SCI (at least one year post-onset), neurological level of injury C3 and below, from traumatic or non-traumatic causes, including tetraplegia and paraplegia, irrespective of sex, race, ethnicity or socio-economic status.
The guidelines may be appropriate for individuals with a SCI less than one year post-onset, aged 65 years or older, or living with comorbid conditions. There is currently insufficient scientific evidence to draw firm conclusions about the risks and benefits of the guidelines for these individuals. These individuals should consult a health care provider prior to beginning an exercise programme.
The risks associated with these guidelines are minimal when managed in consultation with a health care professional who is knowledgeable in SCI. Individuals with a cervical or high thoracic injury should be aware of the signs and symptoms of autonomic dysreflexia during exercise.
Refer to our article on Autonomic Dysreflexia for more information!
These guidelines were developed using transparent and rigorous steps that align with international best-practices for developing clinical practice guidelines. They represent an important step toward developing exercise policies and programs for people with SCI around the world.
Hicks, A. L., Martin Ginis, K. A., Pelletier, C. A., Ditor, D. S., Foulon, B., & Wolfe, D. L. (2011). The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord, 49(11), 1103–1127.
Martin Ginis, K. A., van der Scheer, J. W., Latimer-Cheung, A. E., Barrow, A., Bourne, C., Carruthers, P., … Goosey-Tolfrey, V. L. (2018). Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord, 56(4), 308–321.
Hoekstra, F., McBride, C.B., Borisoff, J. et al. (2020). Translating the international scientific spinal cord injury exercise guidelines into community and clinical practice guidelines: a Canadian evidence-informed resource. Spinal Cord. https://doi.org/10.1038/s41393-019-0410-1
Author: SCIRE Community Team | Reviewer: Darryl Caves | Published: 9 April 2018 | Updated: ~
Changes to blood pressure control after spinal cord injury (SCI) may contribute to a condition called orthostatic hypotension. This page provides information about orthostatic hypotension after SCI.
Key Points
Orthostatic hypotension (also called postural hypotension) is a decrease in blood pressure when moving from a sitting or lying position to an upright position.
Orthostatic hypotension is common early after an SCI, but it can also be present long-term or following an illness or period of reduced mobility. Orthostatic hypotension may happen with or without symptoms.
Orthostatic hypotension is more common early after injury. For some people, orthostatic hypotension gradually goes away as their body adapts to the changes after the injury. However, for others, orthostatic hypotension persists over time and may require treatment to manage their symptoms.
Orthostatic hypotension can happen to anyone after SCI; however certain factors are associated with developing orthostatic hypotension.
Orthostatic hypotension is more common in people with higher levels of SCI, especially with injuries in the cervical and mid to upper thoracic spinal cord (injuries above T6).
People with traumatic SCI are more likely to develop orthostatic hypotension than those with a non-traumatic SCI.
When the body moves into an upright position, the blood naturally flows downward because of gravity. If the body does not respond in any way to help push the blood back up, it pools in the blood vessels of the lower body, causing a drop in blood pressure in the upper body. If blood pressure is low, not enough blood can reach the heart and brain, which causes symptoms like dizziness, fainting and tiredness.
Under normal circumstances, when we move upright, the body’s blood pressure control mechanisms kick in to maintain circulation. The main changes include:
The combined narrowing of blood vessels and increase in heart rate help maintain blood pressure against the force of gravity in standing. These changes are controlled by the autonomic nervous system.
Orthostatic hypotension is common after SCI, particularly in the early period after injury. There are several reasons that orthostatic hypotension happens after SCI.
During the early phase after injury, bed rest is often required for healing of injuries. However, long periods of bed rest and reduced movement can cause the heart and blood vessels to become less responsive to position changes and changes in blood pressure.
During bed rest, there is a shift in the distribution of fluids within the body which causes a loss of fluid through urination (called diuresis). This leads to reduced blood volume (the volume of blood in circulation), which affects the body’s ability to adapt to changes in blood pressure. This likely contributes to orthostatic hypotension in the early phase after SCI.
The body may also produce more of a chemical called nitric oxide as a result of bed rest following SCI, which causes widening (relaxing) of the blood vessels and further contribute to orthostatic hypotension.
The main reason that orthostatic hypotension happens after SCI is because of altered control of the autonomic nervous system.
The autonomic nervous system controls largely unconscious bodily processes such as blood pressure, heart rate, breathing rate, body temperature, digestion, bladder, bowel and sexual function. It has two divisions:
The sympathetic and parasympathetic systems have different (and often opposite) effects on the organs and work together to control bodily functions according to the situation.
Most of the nerves that control the sympathetic nervous system arise from the thoracic spinal cord. Because the sympathetic nerves are responsible for narrowing (constricting) the blood vessels and increasing heart rate when changing positions, SCI in these areas can affect blood pressure control. Without the sympathetic nervous system, the blood vessels and heart rate do not respond appropriately to changes in position and blood can pool in the legs and abdomen, leading to orthostatic hypotension.
Loss of muscle activity in the legs and trunk after SCI may also contribute to orthostatic hypotension. Normally, when the muscles tense, they act like small pumps that squeeze the blood vessels to help push blood back up to the heart. Muscle activity in the legs and abdomen helps to maintain blood pressure in standing. This is sometimes called the skeletal muscle pump.
If there is paralysis in the leg muscles, the muscles do not help to pump blood back up to the heart, which can lead to blood pooling and orthostatic hypotension. This is why sometimes just sitting for a long time after a position change to upright can lead to symptoms of orthostatic hypotension. The effects of the loss of muscle activity seem to have a greater impact earlier after injury, before the body develops strategies to compensate for blood pressure changes.
 During the early phase after injury, bed rest is often required for healing of injuries. However, long periods of bed rest and reduced movement can cause the heart and blood vessels to become less responsive to position changes and changes in blood pressure. This likely contributes to orthostatic hypotension in the early phase after SCI.
During the early phase after injury, bed rest is often required for healing of injuries. However, long periods of bed rest and reduced movement can cause the heart and blood vessels to become less responsive to position changes and changes in blood pressure. This likely contributes to orthostatic hypotension in the early phase after SCI.
The body may also produce more of a chemical called nitric oxide as a result of bed rest following SCI, which causes widening (relaxing) of the blood vessels and further contributes to orthostatic hypotension.
The body regulates blood volume (the amount of blood in circulation) through water and salt levels in the blood. Spinal cord injury can disrupt this balance and lead to low blood volume, low blood pressure and orthostatic hypotension. Low salt levels in the blood are also common after SCI, which can also contribute to orthostatic hypotension. A diet that is low in salt or fluids can also contribute to orthostatic hypotension.
Warm environments can increase the likelihood of experiencing orthostatic hypotension. The body responds to heat by widening the blood vessels, which lowers blood pressure. Excessive sweating in warm environments may also lead to dehydration, which further contributes to orthostatic hypotension.
 Medications
MedicationsMany medications can trigger orthostatic hypotension. These include medications used to treat conditions such as high blood pressure, heart disease and erectile dysfunction.
Consuming alcohol and caffeine can worsen orthostatic hypotension by affecting the constriction of blood vessels during position changes and contributing to dehydration.
After eating, more blood is sent to the intestines for digestion, which may lower blood pressure. Postprandial hypotension is a form of orthostatic hypotension experienced after eating large meals. It is more common among older people.
People with complete cervical SCI may experience orthostatic hypotension during and after exercise, even if they are well-hydrated and have taken steps to avoid overheating. This happens because the heart and blood vessels do not respond adequately to compensate for the increased blood flow to the muscles. This can cause symptoms like dizziness or nausea while exercising. To adjust for this, individuals may start their exercise programs with a slow progression or may need to use other strategies to enable them to exercise safely.
To diagnose orthostatic hypotension, blood pressure is first measured while in a lying or sitting position and then again in an upright or standing position. The two blood pressure readings are compared to see if there is a drop in blood pressure. Other times, severe symptoms alone (such as fainting) may be used to diagnose orthostatic hypotension.
Since many people with SCI cannot stand, blood pressure can be assessed based on a lying then sitting position instead. A tilt table or other supportive device can be used to assist with getting upright.
The heart contracts and relaxes as it pumps blood throughout the body. A blood pressure reading usually shows up with two numbers:
Orthostatic hypotension is diagnosed when there is a decrease of at least 20 mmHg in systolic blood pressure or a decrease of at least 10 mmHg in diastolic blood pressure after moving from lying or sitting to standing.
Currently, midodrine hydrochloride (midodrine) is the only medication recommended for managing orthostatic hypotension after SCI. There is not enough evidence to support the use of other medications. However, medications intended to help treat orthostatic hypotension may also have other unwanted effects on blood pressure after SCI, so should be discussed in detail with your health team for more information.
Midodrine hydrochloride (midrodrine, ProAmatine) is a medication that causes the blood vessels to tighten (constrict). When this occurs, more blood flow travels to the heart and blood pressure increases to prevent blood pressure from dropping in standing. Midodrine is taken by mouth in the form of tablets. Regular blood pressure monitoring done by you or your caregiver will be required to ensure the medication is working properly.
There is moderate evidence that midodrine is effective for treating orthostatic hypotension after SCI and may improve exercise performance by decreasing symptoms of low blood pressure.
There are a number of other medications that have been suggested for reducing orthostatic hypotension, but are ineffective or the evidence is inconclusive. These include:
Several other treatment options may be used to treat orthostatic hypotension. Although there is limited evidence to determine whether these treatments are effective, they may be used in addition to drug treatments to help manage orthostatic hypotension after SCI.
Adequate fluid and salt intake may help to increase blood volume and maintain blood pressure. There is weak evidence that fluid and salt intake combined with medications may be effective for treating orthostatic hypotension after SCI. However, no research studies have been done using fluid and salt alone.
Blood pooling happens when gravity causes blood to collect in the abdomen and legs. This limits blood flow to other areas of the body and contributes to orthostatic hypotension. Compression garments are devices that apply pressure to these areas of the body to help blood circulate back up toward the heart. Compression garments used after SCI include:
Compression garments are commonly used for orthostatic hypotension after SCI because they are inexpensive and unlikely to cause side effects. However, there is conflicting evidence about whether they are effective to reduce orthostatic hypotension after SCI.
Click here to read more about Abdominal Binders.
After a prolonged period of bed rest (such as very early after an SCI) it may take time for the body to adjust to getting upright. Health providers will usually gradually progress a person towards getting upright to help adjust the body to the new position slowly.
This may be done by dangling the legs on the edge of the bed for a period of time before slowly raising the head and getting into a wheelchair. It may also be done by gradually increasing the degree of incline of a tilt table while being mindful of symptoms.
Gradual progression towards moving upright is commonly used in the management of orthostatic hypotension, however, it has not been investigated in research studies.
Regular exercise improves blood flow and may enhance the body’s ability to adapt to changes in posture. However, after an SCI, there are many different ways that the body responds to exercise, depending on the characteristics of the injury itself. People who have complete injuries in the cervical spinal cord can actually sometimes cause a drop in blood pressure (see above).
There is moderate evidence that arm exercise is not beneficial for reducing orthostatic hypotension in people with SCI.
Standing treatments, especially those that involve some type of stimulation to the leg muscles, are thought to stimulate the nervous system and potentially improve blood pressure responses to standing. There is weak evidence that standing with a harness and assistance from health providers may help to increase resting blood pressure and improve orthostatic hypotension in people with cervical SCI.
See our article on Supported Standing for more information!
Functional electrical stimulation (FES) is a treatment where electrical stimulation is applied to the nerves and muscles to cause muscle contractions during an activity. Functional electrical stimulation stimulates the nerves and produces muscular contractions, which may help pump blood back to the heart. There is moderate evidence that functional electrical stimulation can help stabilize blood pressure during changes in position and may be used to supplement other forms of orthostatic hypotension treatment after SCI.
See our article on FES for more information!
Whole-body vibration is a primarily experimental treatment that involves exercising on a vibrating platform. This treatment is thought to cause muscle contractions which may help improve blood flow. There is strong evidence that whole-body vibration can increase blood pressure in people with SCI, but the direct effects of vibration on orthostatic hypotension after SCI has not been studied. Currently, whole-body vibration is not usually available outside of research settings.
Orthostatic hypotension is a common condition after SCI, in which blood pressure drops when moving from a lying or sitting position to an upright position. For some, orthostatic hypotension gradually goes away over time. For others, it may persist long-term and require medical treatment.
While research evidence supports the use of the medication midodrine for treating orthostatic hypotension following SCI, there is little evidence to support the use of other medications for this purpose.
Many non-drug treatments also exist that are commonly used. However, for the most part, there is a lack of research on whether these treatments are effective. Functional electrical stimulation may be effective in reducing orthostatic hypotension after SCI.
It is important to discuss these treatment options with your health providers to determine which ones are suitable options for you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Professional “Orthostatic Hypotension” Module:
Krassioukov A, Wecht JM, Teasell RW, Eng JJ (2014). Orthostatic Hypotension Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p. 1-26.
Available from: scireproject.com/evidence/orthostatic-hypotension/
Midodrine:
[1] Nieshoff EC, Birk TJ, Birk CA, Hinderer SR, Yavuzer G. Double-blinded, placebo-controlled trial of midodrine for exercise performance enhancement in tetraplegia: a pilot study. J Spinal Cord Med 2004;27:219-225.
[2] Wecht JM, Rosado-Rivera D, Handrakis JP, Radulovic M, Bauman WA. Effects of midodrine hydrochloride on blood pressure and cerebral blood flow during orthostasis in persons with chronic tetraplegia. Arch Phys Med Rehabil 2010; 91: 1429-1435.
Other medications:
[1] Barber DB, Rogers SJ, Fredrickson MD, Able AC. Midodrine hydrochloride and the treatment of orthostatic hypotension in tetraplegia: two cases and a review of the literature. Spinal Cord 2000;38:109-111.
[2] Groomes TE, Huang CT. Orthostatic hypotension after spinal cord injury: treatment with fludrocortisone and ergotamine. Arch Phys Med Rehabil 1991;72:56-58.
[3] Frisbie JH, Steele DJ. Postural hypotension and abnormalities of salt and water metabolism in myelopathy patients. Spinal Cord 1997;35:303-307.
[4] Wecht JM, Rosado-Rivera D, Weir JP, Ivan A, Yen C, Bauman WA. Hemodynamic effects of L-Threo-3,4-dihydroxyphenylserine (droxidopa) in hypotensive individuals with spinal cord injury. Arch Phys Med Rehabil 2013; 94: 2006-2012.
[5] Muneta S, Iwata T, Hiwada K, Murakami E, Sato Y, Imamura Y. Effect of L-threo-3, 4-dihydroxyphenylserine on orthostatic hypotension in a patient with spinal cord injury. Jpn Circ J 1992;56:243-247.
[6] Wecht JM, Radulovic M, Rosado-Rivera D, Zhang RL, La Fountaine MF, Bauman WA. Orthostatic effects of midodrine versus L-NAME on cerebral blood flow and the renin-angiotensin-aldosterone system in tetraplegia. Arch Phys Med Rehabil 2011; 92: 1789-1795.
[7] Wecht JM, Weir JP, Goldstein DS, Krothe-Petroff A, Spungen AM, Holmes C, et al. Direct and reflexive effects of nitric oxide synthase inhibition on blood pressure. Am J Physiol Heart Circ Physiol 2008;294:H190-197.
[8] Wecht JM, Radulovic M, La Fountaine M, Rosado-Rivera D, Zhang RL, Bauman W. Orthostatic responses to nitric oxide synthase inhibition in persons with tetraplegia. Arch Phys Med Rehabil; 2009:1428-1434.
[9] Wecht JM, Weir JP, Krothe AH, Spungen AM, Bauman WA. Normalization of supine blood pressure after nitric oxide synthase inhibition in persons with tetraplegia. J Spinal Cord Med 2007; 30: 5-9.
[1] Yarar-Fisher C, Pascoe DD, Gladden LB, Quindry JC, Hudson J, Sefton J. Acute physiological effects of whole body vibration (WBV) on central hemodynamics, muscle oxygenation and oxygen consumption in individuals with chronic spinal cord injury. Disabil Rehabil 2013; early online: 1-10.
[2] Faghri PD, Yount J. Electrically induced and voluntary activation of physiologic muscle pump: a comparison between spinal cord-injured and able-bodied individuals. Clin Rehabil 2002;16:878-885.
[3] Elokda AS, Nielsen DH, Shields RK. Effect of functional neuromuscular stimulation on postural related orthostatic stress in individuals with acute spinal cord injury. J Rehabil Res Dev 2000;37:535-542.
[4] Sampson EE, Burnham RS, Andrews BJ. Functional electrical stimulation effect on orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil 2000; 81: 139-143.
[5] Lopes P, Figoni SF, Perkash I. Upper limb exercise effect on tilt tolerance during orthostatic training of patients with spinal cord injury. Arch Phys Med Rehabil 1984;65:251-253.
[6] Ditor DS, Macdonald MJ, Kamath MV, Bugaresti J, Adams M, McCartney N, Hicks AL. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 2005;43:664-673.
[7] Otsuka Y, Shima N, Moritani T, Okuda K, Yabe K. Orthostatic influence on heart rate and blood pressure variability in trained persons with tetraplegia. Eur J Appl Physiol 2008;104:75-78.
[8] Harkema SJ, Ferreira CK, van den Brand RJ, Krassioukov AV. Improvements in orthostatic instability with stand locomotor training in individuals with spinal cord injury. J Neurotrauma 2008;25:1467-1475.
Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 2006;44:341-351.
Hopman MT, Groothuis JT, Flendrie M, Gerrits KH, Houtman S. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol 2002;93:1966-1972.
McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 1999;80:1402-1410.
Munakata M, Kameyama J, Kanazawa M, Nunokawa T, Moriai N, Yoshinaga K. Circadian blood pressure rhythm in patients with higher and lower spinal cord injury: simultaneous evaluation of autonomic nervous activity and physical activity. J Hypertens 1997;15:1745-1749.
Narkiewicz K, Cooley RL, Somers VK. Alcohol potentiates orthostatic hypotension: Implications for alcohol-related syncope. Circulation 2000; 101: 398-402.
Vaziri ND. Nitric oxide in microgravity-induced orthostatic intolerance: relevance to spinal cord injury. J Spinal Cord Med 2003;26:5-11.
Wecht JM, Bauman WA. Implication of altered autonomic control for orthostatic tolerance in SCI. Auton Neurosci. 2018 Jan;209:51-58.
Wecht JM, De Meersman RE, Weir JP, Spungen AM, Bauman WA. Cardiac autonomic responses to progressive head-up tilt in individuals with paraplegia. Clin Auton Res 2003; 13: 433-438.
Author: SCIRE Community Team | Reviewer: Darryl Caves | Published: 17 January 2018 | Updated: ~
Standing with supportive equipment is a therapy option after spinal cord injury (SCI). This page outlines basic information about the use of supported standing after SCI.
Key Points
Standing is an important part of functional movement in humans. Standing is needed for walking, and also provides a challenge to the circulatory system, bones, and muscles in ways that cannot be achieved in sitting or lying.
Passive standing (standing with support instead of by muscle activation) may have treatment benefits after SCI, even when the recovery of walking and standing abilities is unlikely. Standing may have benefits in treating health conditions associated with SCI, such as conditions involving the musculoskeletal, circulatory, breathing, bowel and bladder systems. It remains a key treatment tool used in rehabilitation.
Supported standing involves the use of special equipment to support an upright posture. The type of equipment used depends on the person’s unique characteristics and abilities (such as the amount of muscle control in the arms, legs and trunk), the equipment that is available, and other medical concerns like joint contractures, spasticity and osteoporosis. Equipment used for standing may include a wide range of different devices such as:
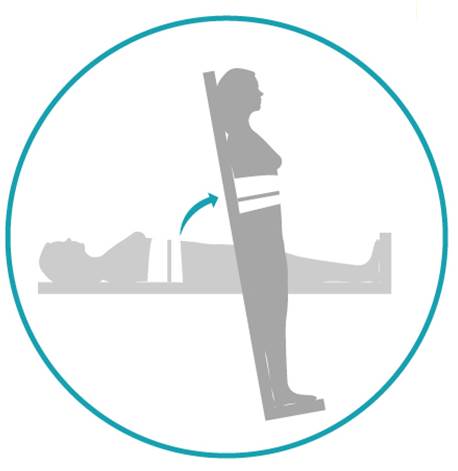
Tilt tables are flat surfaces that can be tilted from a horizontal position into a vertical standing position. The person is strapped securely to the table while in a horizontal lying position and the table can be tilted vertically. Tilt tables are typically the first devices that are used to work towards standing because the table can be gradually increased by degrees. This is often needed because it may take some time to tolerate being upright and maintaining a safe blood pressure. It is also a good device to test a person’s physical tolerance and safety for standing.
Standing frames are simple frames that have padding at the joints to support a standing position. There are many different types of standing frames. The frame needs to be fitted to the person’s unique physical abilities and body type and minimize areas of excess pressure.
Standing wheelchairs are wheelchairs that can extend from a sitting position into standing. There are many different types of standing wheelchairs, from manual devices to motorized systems. However, standing wheelchairs are expensive and not commonly available.
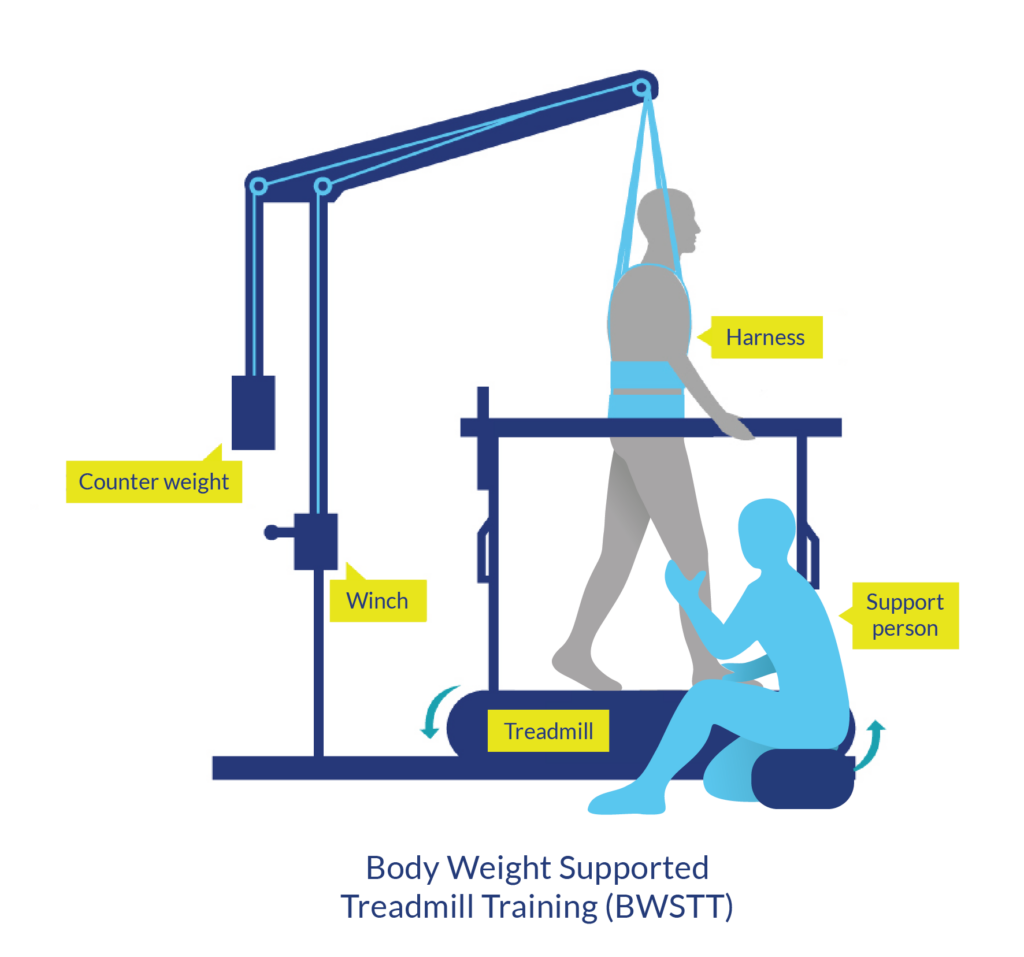
Suspension body weight support systems involve a harness system that is suspended from above to support a percentage of body weight while standing. These systems are typically used while walking on a treadmill (body weight-supported treadmill training) or sometimes while walking over ground. This type of system is usually used for people with incomplete injuries that may work towards standing or stepping independently.
See our article on Body Weight Supported Treadmill Training.
Robotic exoskeletons are a relatively new and emerging technology that is typically used for walking and walking training, but may also have benefits related to standing. However, this equipment is costly and not available in most settings.
Walkers, crutches or canes may be used by people with incomplete SCI and good strength in the arms who need only minimal support in standing.
Orthoses and braces may be used to brace the hip, knee and/or ankle joints to keep them from bending. This can help to support the person in an upright standing posture with training and rehabilitation. Orthoses and braces are typically custom-made and usually used by people with paraplegia who have good upper body strength and hip flexibility. Orthoses and braces used for standing may include:
Functional electrical stimulation (FES) involves the use of electrical stimulation to activate muscles that are weak or paralyzed after an SCI during a purposeful activity. FES over the trunk or leg muscles may be used while standing with equipment for added benefits.
See our article on Functional Electrical Stimulation (FES) for more information.
Standing equipment may be expensive and sometimes requires repeated visits to healthcare facilities, which can sometimes be a barrier to regular standing. It is important for the individual to work with their health providers to find appropriate equipment that is safe and suitable.
Once appropriate equipment and strategies for standing are selected with assistance from a health provider, standing is gradually introduced over time. The amount of time spent standing, the amount of load that is taken through the legs, and the final standing position will be adjusted until a suitable standing position can be maintained. During these first several sessions, health providers will monitor for any adverse effects related to the treatment.
Current research findings are unable to tell us how long or how often standing should be done to have benefits. Studies have used standing for 20 to 60 minutes, three to four times per week to study the effects of this treatment. It will be different for everyone. The standing prescription will be based on the person’s unique situation.
Depending on the treatment goals, standing may also involve:
Supported standing is considered to be a relatively safe treatment for use after SCI. However, there are some situations in which standing may not be appropriate and some possible risks. This is not a complete list; please consult a health provider for further safety information.
For more information on these topics, see our articles on Pressure Injuries, Orthrostatic Hypotension, Osteoporosis, Spasticity, and Autonomic Dysreflexia
If using electrical stimulation, the safety precautions and risks associated with use of functional electrical stimulation (FES) also apply.
It is not clear if standing helps to maintain or increase bone density in the legs after SCI. Current research evidence is inconclusive and further studies are needed.
Another proposed use of standing after SCI is to help with blood pressure control. One study provides weak evidence that standing with a harness and assistance from health providers helps to increase resting blood pressure and reduce drops in blood pressure when standing (orthostatic hypotension) in people with cervical SCI.
There is weak evidence that standing may help to reduce spasticity short term in people with SCI. There are also surveys that report that many people with SCI report that regular standing helped to reduce their spasticity.
There is not enough evidence to determine whether standing can also improve bowel function. Further research in this area is needed.
Supported standing serves as a therapy option for people such as individuals with SCI as they do not normally stand as part of their everyday mobility. This therapy involves equipment such as standing frames, tilt tables, orthoses, or standing wheelchairs to support an upright position for a designated period of time. While there is research supporting the benefits of supported standing, conflicting evidence is still apparent. Further research is needed to better understand the benefits of supported standing after SCI.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Professional “Bone Health”, “Orthostatic Hypotension”, “Spasticity”, and “Bowel Dysfunction and Management” Modules:
Craven C, Lynch CL, Eng JJ (2014). Bone Health Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 37.
Available from: https://scireproject.com/evidence/bone-health/
Krassioukov A, Wecht JM, Teasell RW, Eng JJ (2014). Orthostatic Hypotension Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p. 1-26.
Available from: https://scireproject.com/evidence/orthostatic-hypotension/
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Curt A, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0.
Available from: https://scireproject.com/evidence/spasticity/
Coggrave M, Mills P, Willms R, Eng JJ, (2014). Bowel Dysfunction and Management Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 48.
Available from: https://scireproject.com/evidence/bowel-dysfunction-and-management/
[1] Dudley-Javoroski S, Saha PK, Liang G, Li C, Gao Z, and Shields RK. High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos Int 2012; 23:2335-2346.
[2] Goktepe A, Tugco I, Alaca, R, Gunduz S, Nikent M. Does standing protect bone density in patients with chronic spinal cord injury? JSCM 2008;31:197-201.
[3] Needham-Shropshire BM, Broton JG, Klose KJ, Lebwohl N, Guest RS, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 3. Lack of effect on bone mineral density. Arch Phys Med Rehabil 1997;78:799-803.
[4] Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993;74:73-78.
[5] Kaplan PE, Roden W, Gilbert E, Richards L, Goldschmidt JW. Reduction of hypercalciuria in tetraplegia after weight-bearing and strengthening exercises. Paraplegia 1981;19:289-293.
[6] Ben M, Harvey L, Denis S, et al. Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Aust J Physiother. 2005;51:251-256.
[7] de Bruin ED, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stussi E. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil 1999;80:214-220.
[8] Dudley-Javoroski S, and Shields RK. Active-resisted stance modulates regional bone mineral density in humans with spinal cord injury. Journal of Spinal Cord Medicine 2013; 36: 191-199.
[1] Harkema SJ, Ferreira CK, van den Brand RJ, Krassioukov AV. Improvements in orthostatic instability with stand locomotor training in individuals with spinal cord injury. J Neurotrauma 2008;25:1467-1475.
[1] Odeen I, Knutsson E. Evaluation of the effects of muscle stretch and weight load in patients with spastic paraplegia. Scand J Rehabil Med 1981;13:117-21.
[2] Bohannon R. Tilt table standing for reducing spasticity after spinal cord injury. Arch Phys Med Rehabil 1993;74:1121-2.
[3] Kunkel C, Scremin A, Eisenberg B, Garcia J, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993;74:73-8.
[4] Dunn R, Walter J, Lucero Y, Weaver F, Langbein E, Fehr L, et al. Follow-up assessment of standing mobility device users. Assist Technol 1998;10:84-93.
[5] Eng JJ, Levins S, Townson A, Mah-Jones D, Bremner J, Huston G. Use of prolonged standing for individuals with spinal cord injuries. Phys Ther 2001;81:1392-9.
[6] Shields R & Dudley-Javoroski S. Monitoring standing wheelchair use after spinal cord injury: a case report. Disabil Rehabil 2005;27:142-6.
[1] Hoenig H, Murphy T, Galbraith J, Zolkewitz M. Case study to evaluate a standing table for managing constipation. SCI Nursing 2001;18:74-7.
Dunn RB, Walter JS, Lucero Y, Weaver F, Langbein E, Fehr L, Johnson P, Riedy L. Follow-up assessment of standing mobility device users. Assist Technol. 1998;10(2):84-93.
Glickman LB, Geigle PR, Paleg GS. A systematic review of supported standing programs. J Pediatr Rehabil Med. 2010; 3(3),197-213.
Sadeghi M, McLvor J, Finlayson H, Sawatzky B. Static standing, dynamic standing and spasticity in individuals with spinal cord injury. Spinal Cord 2016;54:376-82.
Spinal Cord Injury Centre Physiotherapy Lead Clinicians. Clinical guideline for standing adults following spinal cord injury. 2019 Apr. Available from: https://scireproject.com/wp-content/uploads/2023/01/SCI-Standing-Clinical-Guidelines-Report-2019.pdf
Author: SCIRE Community Team | Reviewers: Tania Lam, Shannon Sproule | Published: 29 November 2017 | Updated: ~
Body weight supported treadmill training is a therapy that can be used to support walking training after spinal cord injury (SCI). This page outlines basic information about the use of body weight supported treadmill training after SCI.
Key Points
Body weight supported treadmill training is a therapy modality in which part of a person’s body weight is supported while walking on a treadmill. It is usually done using an overhead suspension system attached to a harness that supports part of a person’s body weight over a treadmill. While supported, the person walks with or without assistance from health providers on a treadmill.

Man engages in body weight supported treadmill training.
Body weight supported treadmill training is usually used to work on walking in people with some control of movement in their legs after SCI (usually people with incomplete SCI).
To practice walking and standing
Body weight supported treadmill training is usually used to work on walking and standing skills after incomplete SCI. Because the body weight is partially supported, walking can be practiced even when a person cannot stand or walk independently. This may also allow for walking training to begin earlier after injury.
To work on walking quality and speed
Body weight supported treadmill training may be used to practice better walking patterns and prevent unwanted movement compensations that can happen during unsupported walking. It may also allow a person to safely practice walking at faster speeds. This may provide important feedback to the nervous system to help with learning.
To train fitness and health
Standing upright and walking may have benefits for cardiovascular fitness and overall health. It may also have other benefits, such as improving spasticity and feelings of wellness.
Body weight supported treadmill training usually involves the use of an overhead harness and suspension system that supports the person in standing over a treadmill. There are other forms of body weight support training, such as underwater treadmills, anti-gravity treadmills and robotic assisted systems, although these are less common in standard clinical settings.
The amount of body weight that is supported will be different for each person depending on the characteristics of their SCI (such as the level of injury), the level of support provided by the health providers, and the person’s experience with the training.

Equipment for a suspension type system includes a treadmill, overhead suspension system, and harness1
Body weight supported treadmill training may involve the use of several different pieces of equipment, depending on the type of support provided. The most common type of harness and suspension system may involve a variety of pieces of equipment such as:
Some body weight supported treadmill training systems may also involve the use of computer systems which control the training and/or robotic systems which guide movement of the legs.
The exact procedures depend on the type of equipment used and the person’s physical abilities. General procedures for use of a standard harness and overhead suspension system may include the following steps:

Female clinician adjusting the harness, preparing a man for the treadmill.
Training usually begins with maximum body weight support at a slow speed. The amount of support is usually between 35% and 50% of body weight, but depends on your ability to stand on one leg without it buckling. As you get used to the training, the amount of support provided is reduced and the speed or time spent on the treadmill can be increased. It is important to maintain a good quality walking pattern to practice normal movement patterns.
Body weight supported treadmill training is usually done using an overhead suspension system and harness that supports the body over a treadmill. Hands-on assistance or braces may be used to help move the legs or control the trunk and pelvis.
Your health provider will determine how long the training will last, depending on you and your training goals, as well as the availability of equipment and staff. Body weight supported treadmill training is often done for 15 to 30 minutes two to five times per week. However, we do not know what the optimal amount of training is.
Body weight supported treadmill training is just one of many different walking therapies for people with SCI. It is often accompanied by other forms of walking training such as:
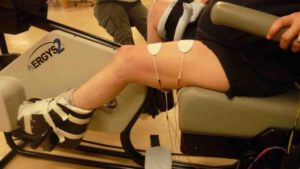
FES training can also strengthen muscles used for walking
It is important to speak with a health provider about body weight supported treadmill training to make sure it is safe and suitable for you and to learn how to use the equipment correctly.
Refer to our article on Functional Electrical Stimulation (FES) for more information!
There are some situations in which body weight supported treadmill training may be unsafe to use. This not a complete list, speak to a health provider about whether this treatment is safe and appropriate for you.
There are some risks and side effects that should be discussed before participating in body weight supported treadmill training. This is not a complete list; ask your health providers for more detail.
In addition to the risks and side effects of body weight supported treadmill training, there are also practical limitations its use, including:
Research studies have found that body weight supported treadmill training may help to:

However, the benefits for walking do not appear to be unique to this type of training. Most walking strategies which involve weight-bearing (including walking overground, treadmill walking, and walking with FES) appear to be equally effective at improving walking after incomplete SCI.
Several studies have looked at the effects of body weight supported treadmill training on different aspects of cardiovascular fitness after SCI. Taken altogether, these studies provide early evidence that body weight supported treadmill training helps to improve many aspects of cardiovascular fitness and health in people with complete and incomplete tetraplegia and paraplegia.
In addition to benefits for walking and fitness, body weight supported treadmill training may also have other effects after SCI.
Although we tend to think about walking as being entirely voluntary, the ability to step and walk is actually related to both conscious and unconscious (automatic) processes. Some of the automatic walking processes are thought to be controlled within the spinal cord by networks of nerve cells known as central pattern generators or CPGs.
Central pattern generators (CPGs or spinal pattern generators) are networks of nerve cells in the spinal cord that generate rhythmic movement patterns. These networks do not require signals from the brain or sensation to keep going once they are activated.
CPGs were discovered when researchers found that animals with complete SCI demonstrated stepping movements when they were supported over a treadmill. These animals could not start the movement themselves, but once it was triggered (typically by electrical stimulation, application of certain drugs, or sensory stimulation to an area between the pubic bone and sacrum called the perineum), the stepping movements continued in a rhythmic pattern which resembled walking.
These networks of nerve connections are thought to be located within the spinal cord itself and exist to allow repetitive movements to continue without the need to think about each step.
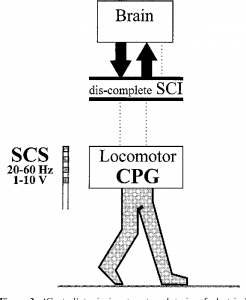
Generation of rhythmic movement through the CPG is debatable in people with SCI2
Researchers are still unsure about whether central pattern generators can be activated after complete SCI in humans. Researchers have suggested several observations that may show evidence of central pattern generators after complete SCI in humans, including:
However, there is debate among researchers about whether these findings really show evidence of central pattern generators or not. It is also not clear if central pattern generators are activated during body weight supported treadmill training after SCI.
Refer to our article on Epidural Stimulation for more information!
It is also important to consider that automatic stepping is not walking. Walking is much more complex, involving many other components, such as strength to support the body weight, balance to stay upright and shift weight, and sensation and voluntary control to adapt to the environment and situation. For these reasons, even if central pattern generators are activated after complete SCI, we do not know whether this will help a person regain walking ability or have any other benefits for functional walking.
Further research is needed to better understand central pattern generators after complete SCI. At this time, body weight supported treadmill training continues to be used clinically as a treatment for people with incomplete SCI who retain some movement in the legs.
Overall, the research evidence suggests that body weight supported treadmill training has positive effects on walking after incomplete SCI that are similar to other forms of walking training. It may also have benefits for fitness, spasticity, and wellness after SCI, although more high quality research is needed to confirm.
Body weight supported treadmill training appears to be relatively safe when used appropriately, however the equipment and support needed for this treatment may not be commonly available for regular use. If you are interested in this treatment, discuss your options with your health providers to find out if it is suitable to you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Lam T, Wolfe DL, Domingo A, Eng JJ, Sproule S (2014). Lower Limb Rehabilitation Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-74.
Available from: https://scireproject.com/evidence/lower-limb-and-walking/
Warburton DER, Krassioukov A, Sproule S, Eng JJ (2014). Cardiovascular Health and Exercise Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-48.
Available from: https://scireproject.com/evidence/cardiovascular-health-and-exercise/
Craven C, Lynch CL, Eng JJ (2014). Bone Health Following Spinal Cord Injury, In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-37.
Available from: https://scireproject.com/evidence/bone-health/
Orenczuk S, Mehta S, Slivinski J, Teasell RW (20140). Depression Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-35.
Available from: https://scireproject.com/evidence/mental-health/depression/
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Curt A, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0.
Available from: https://scireproject.com/evidence/spasticity/
Evidence for “Does body weight supported treadmill training improve walking after incomplete SCI?” is based on the following studies:
Walking
[1] Behrman AL, Ardolino E, VanHiel LR, Kern M, Atkinson D, Lorenz DJ, Harkema SJ. Assessment of functional improvement without compensation reduces variability of outcome measures after human spinal cord injury. Arch Phys Med Rehabil 2012;93:1518-29.
[2] Buehner JJ, Forrest GF, Schmidt-Read M, White S, Tansey K, Basso DM. Relationship between ASIA examination and functional outcomes in the NeuroRecovery Network Locomotor Training Program. Arch Phys Med Rehabil 2012;93:1530-40.
[3] Lorenz DJ, Datta S, and Harkema SJ. Longitudinal patterns of functional recovery in patients with incomplete spinal cord injury receiving activity-based rehabilitation. Arch Phys Med Rehabil 2012;93:1541-52.
[4] Winchester P, Smith P, Foreman N, Mosby J, Pacheco F, Querry R, and Tansey K. A prediction model for determining over ground walking speed after locomotor training in persons with motor incomplete spinal cord injury. J of Spinal Cord Med 2009;32:63-71.
[5] Hicks AL, Adams MM, Martin Ginis K, Giangregorio L, Latimer A, Phillips SM, and McCartney N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 2005;43:291-298.
[6] Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, and Hornby TG. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: A multicenter trial. Arch Phys Med Rehabil 2005;86:672-680.
[7] Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 2005;94:2844-2855.
[8] Protas EJ, Holmes SA, Qureshy H, Johnson A, Lee D, and Sherwood AM. Supported treadmill ambulation training after spinal cord injury: a pilot study. Arch Phys Med Rehabil 2001;82:825-831.
[9] Wernig A, Nanassy A, and Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord 1998;36:744-749.
[10] Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: A randomized clinical trial. Physical Therapy 2011;91:48-60.
[11] Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, and Scott M. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 2006;66:484-493.
[12] Hitzig SL, Craven BC, Panjwani A, Kapadia N, Giangregorio LM, Richards K, Masani K, and Popovic MR. Randomized trial of functional electrical stimulation therapy for walking in incomplete spinal cord injury: effects on quality of life and community participation. Top Spinal Cord Inj Rehabil 2013;19(4):245-58.
[13] Field-Fote EC, Lindley SD, and Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther 2005;29:127-137.
[14] Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther 2002;82:707-715.
[15] Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001;82:818-824.
[16] Hesse S, Werner C, and Bardeleben A. Electromechanical gait training with functional electrical stimulation: case studies in spinal cord injury. Spinal Cord 2004;42:346-352.
Cardiovascular fitness
[1] Miller PJ, Rakobowchuk M, Adams MM, Hicks AL, McCartney N, Macdonald MJ. Effects of short-term training on heart rate dynamics in individuals with spinal cord injury. Auton Neurosci 2009;150(1-2):116-21.
[2] Jack LP, Allan DB, Hunt KJ. Cardiopulmonary exercise testing during body weight supported treadmill exercise in incomplete spinal cord injury: a feasibility study. Technol Health Care 2009; 17(1):13-23.
[3] Soyupek F, Savas S, Ozturk O, Ilgun E, Bircan A, Akkaya A.E ffects of body weight supported treadmill training on cardiac and pulmonary functions in the patients with incomplete spinal cord injury. J Back Musculoskelet Rehabil 2009; 22(4):213-8.
[4] Ditor DS, Macdonald MJ, Kamath MV, Bugaresti J, Adams M, McCartney N, et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 2005b;43(11):664-73.
Other effects
[1] Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord 2005;43:649-657.
[2] Hicks AL, Adams MM, Martin GK, Giangregorio L, Latimer A, Phillips SM, McCartney N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: Effects on functional walking ability and measures of subjective well-being. Spinal Cord 2005;43:291-8.
[3] Adams M, Hicks A. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med 2011;34:488-94.
Evidence for “Can body weight supported treadmill training cause stepping after complete SCI?” is based on the following studies:
[1] Minassian K, Hofstoetter US, Dzeladini F, Guertin PA, Ijspeert A. The Human Central Pattern Generator for Locomotion. Neuroscientist. 2017 Mar 1:1073858417699790. doi: 10.1177/1073858417699790. [Epub ahead of print]
[2] Kern H, McKay WB, Dimitrijevic MM, Dimitrijevic MR. Motor control in the human spinal cord and the repair of cord function. Curr Pharm Des. 2005;11:1429-1439.
[3] Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626-2634.
[4] Guertin PA. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol. 2013 Feb 8;3:183.
[5] Yang JF, Gorassini M. Spinal and brain control of human walking: implications for retraining of walking. Neuroscientist. 2006 Oct;12(5):379-89.
[6] Illis LS. Is there a central pattern generator in man? Paraplegia. 1995 May;33(5):239-40.
Other references:
Body Weight Support Treadmill Training. Summary by: Lisa Taipalus BScPT, NEO Regional Stroke Best Practice Consultant for Physiotherapy, December 2009 (updated June 2011).
Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med 2011;364(21):2026-2036.
Senthilvelkumar T, Magimairaj H, Fletcher J, Tharion G, George J. Comparison of body weight-supported treadmill training versus body weight-supported overground training in people with incomplete tetraplegia: a pilot randomized trial. Clin Rehabil 2015; 29(1):42-49.
Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 2013;94(11):2297-2308.
Hicks AL, Ginis KA. Treadmill training after spinal cord injury: it’s not just about the walking. J Rehabil Res Dev 2008; 45(2):241-218.
Image credits:
Author: SCIRE Community Team | Reviewer: Andrei Krassioukov and Janice Eng | Published: 12 October 2017 | Updated: ~
Autonomic dysreflexia is a medical emergency that can happen after spinal cord injury (SCI). This page provides an overview of what autonomic dysreflexia is and how it is managed.
For information about emergency treatments, see How is autonomic dysreflexia treated?
Key Points
Autonomic dysreflexia (also called autonomic hyperreflexia) is a potentially life-threatening medical condition that can happen after spinal cord injury. Autonomic dysreflexia involves a sudden rise in blood pressure accompanied by changes in heart rate and other symptoms like headaches and sweating. The blood pressure responses in the body are poorly controlled because of the SCI and can become dangerously high.

Autonomic dysreflexia involves a sudden rise in blood pressure of 20 to 30 mmHg above your normal systolic blood pressure. Since the normal blood pressure of people with SCI is often 20 to 30 mmHg lower than in those without SCI, blood pressure can be in a range that is commonly considered ‘normal’ or ‘slightly elevated’ and still be high for that person.
This rise in blood pressure is usually accompanied by symptoms. Symptoms will be different for everyone and can range from some mild discomfort to life threatening and severe.
 Autonomic dysreflexia can be life threatening when severe. If left untreated, uncontrolled elevated blood pressure can lead to serious conditions like stroke, heart attack, detached retinas, seizures, and even death.
Autonomic dysreflexia can be life threatening when severe. If left untreated, uncontrolled elevated blood pressure can lead to serious conditions like stroke, heart attack, detached retinas, seizures, and even death.
Although these complications are uncommon, it is important that autonomic dysreflexia is recognized and treated immediately. Talk to your health providers about setting up a plan for managing episodes of autonomic dysreflexia as soon as they happen.
Autonomic dysreflexia can be triggered by any strong stimulation below the SCI, including anything that could be considered uncomfortable, irritating, or painful if it could be felt. For example, a wound can trigger autonomic dysreflexia even if it the person does not feel it. Autonomic dysreflexia can also be caused by normal body processes that are strongly stimulating, such as a full bladder or sexual stimulation. The most common triggers are related to the bladder or bowel.
Autonomic dysreflexia is caused by dysfunction of the autonomic nervous system after SCI that leads to poorly controlled blood pressure responses.
The autonomic nervous system controls largely unconscious bodily processes such as blood pressure, heart rate, breathing rate, body temperature, digestion, bladder, bowel, and sexual function. It has two divisions:
The sympathetic nervous system prepares the body for stressful or emergency situations. It is often called the ‘fight or flight’ system, because it prepares the body for action. For example, it increases heart rate and constricts blood vessels.
The parasympathetic nervous system prepares the body for normal, non-emergency situations. It is often called the ‘rest and digest’ system, because it allows the body to restore itself. For example, it slows heart rate and relaxes blood vessels.
The sympathetic and parasympathetic systems have different (and often opposite) effects on the organs and work together to control bodily functions according to the situation.
Blood pressure is carefully controlled by the autonomic nervous system to ensure that circulation works properly. The body monitors blood pressure and makes adjustments to maintain blood pressure within an optimal range. This is done in part by tightening (constricting) or relaxing (dilating) the blood vessels and changing heart rate.
 When the body below the SCI detects strong stimulation, it activates the sympathetic nervous system, causing the constriction of blood vessels in the lower body. This causes blood pressure to rise.
When the body below the SCI detects strong stimulation, it activates the sympathetic nervous system, causing the constriction of blood vessels in the lower body. This causes blood pressure to rise.

Pressure sensors in the arteries then detect the elevated blood pressure and relay this message to the brain. Under normal circumstances, the brain sends signals through the spinal cord and cranial nerves to relax the blood vessels and slow heart rate. This restores blood pressure to normal.
However, when there is an SCI, the signal to relax the blood vessels is blocked from travelling to the lower body. Because of this, the blood vessels remain constricted and the body cannot restore blood pressure to normal. This causes the uncontrolled elevated blood pressure that happens during autonomic dysreflexia.
The level of T6 is important because nerves from this part of the spinal cord constrict a large group of blood vessels in the abdomen called the splanchnic vascular bed. These blood vessels contain a large volume of blood, so when the blood vessels are constricted, it causes the blood pressure to rise significantly. Injuries below T6 do not usually cause enough of a change in blood pressure to cause autonomic dysreflexia.
Autonomic dysreflexia is a medical emergency and needs to be treated immediately. Emergency treatment involves a series of steps to lower blood pressure and remove the cause of the episode. If these steps are unsuccessful, emergency medical treatments are used to try to reduce blood pressure quickly.
If blood pressure returns to normal, continue to monitor your symptoms and blood pressure to make sure they do not return and report the incident to a health provider.
This approach is considered to be the most effective first treatment for autonomic dysreflexia. However, although this procedure is commonly used and recommended by health providers, it is supported primarily by expert opinion rather than evidence from research studies.
 If the steps above do not reduce blood pressure and it remains high (150 mmHg or above), seek emergency medical attention by calling an ambulance or visiting an emergency department. Emergency medical treatments involve the use of medications that rapidly lower blood pressure. The health providers may also run a series of tests to identify the cause of the episode if one has not been found.
If the steps above do not reduce blood pressure and it remains high (150 mmHg or above), seek emergency medical attention by calling an ambulance or visiting an emergency department. Emergency medical treatments involve the use of medications that rapidly lower blood pressure. The health providers may also run a series of tests to identify the cause of the episode if one has not been found.
The most commonly used drugs for autonomic dysreflexia are:
However, there is little research evidence to suggest which medications work best for treating autonomic dysreflexia. Speak to your health providers for more information about medications used in autonomic dysreflexia emergencies.
The most effective way of managing autonomic dysreflexia is to prevent triggering it in the first place. People who get autonomic dysreflexia may be able to take steps to avoid triggering episodes, especially during procedures that are known to cause autonomic dysreflexia.
In order to be able to manage episodes of autonomic dysreflexia, it is important to be able to recognize an episode and know what to do when it happens.
 Bladder problems are the most common triggers of autonomic dysreflexia. Methods that may be used to prevent bladder problems from triggering autonomic dysreflexia include:
Bladder problems are the most common triggers of autonomic dysreflexia. Methods that may be used to prevent bladder problems from triggering autonomic dysreflexia include:
See our article on Urinary Tract Infections to learn more!
 Bowel problems are another common trigger of autonomic dysreflexia. Methods that may be used to prevent bowel problems from triggering autonomic dysreflexia include:
Bowel problems are another common trigger of autonomic dysreflexia. Methods that may be used to prevent bowel problems from triggering autonomic dysreflexia include:
See our article on Bowel Changes after Spinal Cord Injury for more information!
 Pressure ulcers, ingrown toenails, and burns can all be additional causes of autonomic dysreflexia. Methods that may be used to prevent skin problems from triggering autonomic dysreflexia include:
Pressure ulcers, ingrown toenails, and burns can all be additional causes of autonomic dysreflexia. Methods that may be used to prevent skin problems from triggering autonomic dysreflexia include:

Autonomic dysreflexia is a serious medical condition that requires immediate recognition and treatment. First line treatment involves sitting up, removing tight clothing, and removing the cause of episode. This is supported by expert opinion. Emergency medical treatments for episodes of autonomic dysreflexia involve fast acting anti-hypertensive agents such as nifedipine, nitrates, and captopril, although the best treatment to use has not been determined.
It is best to discuss treatment options with your health providers to find out which treatments are suitable for you. For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Krassioukov A, Blackmer J, Teasell RW, Eng JJ (2014). Autonomic Dysreflexia Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors.
Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 35. Available from: http://scireproject.com/evidence/rehabilitation-evidence/autonomic-dysreflexia.
Barton CH, Khonsari F, Vaziri ND, Byrne C, Gordon S, Friis R. T http://scireproject.com/evidence/rehabilitation-evidence/autonomic-dysreflexia The effect of modified transurethral sphincterotomy on autonomic dysreflexia. J Urol 1986;135:83-85.http://scireproject.com/evidence/rehabilitation-evidence/autonomic-dysreflexia-re/
Sidi AA, Becher EF, Reddy PK, Dykstra DD. Augmentation enterocystoplasty for the management of voiding dysfunction in spinal cord injury patients. J Urol 1990;143:83-85.
Perkash I. Transurethral sphincterotomy provides significant relief in autonomic dysreflexia in spinal cord injured male patients: Long-term followup results. J Urol 2007;177:1026-1029.
Ke QS, Kuo HC. Transurethral incision of the bladder neck to treat bladder neck dysfunction and voiding dysfunction in patients with high-level spinal cord injuries. Neuro Uro 2010;29:748-752.
Hohenfellner M, Pannek J, Botel U, Bahms S, Pfitzenmaier J, Fichtner J, et al. Sacral bladder denervation for treatment of detrusor hyperreflexia and autonomic dysreflexia. Urol 2001;58:28-32.
Kutzenberger J. Surgical therapy of neurogenic detrusor overactivity (hyperreflexia) in paraplegic patients by sacral deafferentation and implant driven micturition by sacral anterior root stimulation: methods, indications, results, complications, and future prospects. Acta Neurochir Suppl 2007;97:333-339.
Coggrave MJ, Ingram RM, Gardner BP, Norton CS. The impact of stoma for bowel management after spinal cord injury. Spinal Cord 2012;50:848-852.
Cosman BC, Vu TT. Lidocaine anal block limits autonomic dysreflexia during anorectal procedures in spinal cord injury: a randomized, double-blind, placebo-controlled trial. Dis Colon Rectum 2005;48:1556-1561.
Cosman BC, Vu TT, Plowman BK. Topical lidocaine does not limit autonomic dysreflexia during anorectal procedures in spinal cord injury: a prospective, double-blind study. Int J Colorectal Dis 2002;17:104-108.
Furusawa K, Sugiyama H, Tokuhiro A, Takahashi M, Nakamura T, Tajima F. Topical anesthesia blunts the pressor response induced by bowel manipulation in subjects with cervical spinal cord injury. Spinal Cord 2009;47:144-148.
Cross LL, Meythaler JM, Tuel SM, Cross LA. Pregnancy, labor and delivery post spinal cord injury. Paraplegia 1992;30:890-902.
Hughes SJ, Short DJ, Usherwood MM, Tebbutt H. Management of the pregnant woman with spinal cord injuries. Br J Obstet Gynaecol 1991;98:513-518.
Cross LL, Meythaler JM, Tuel SM, Cross AL. Pregnancy following spinal cord injury. West J Med 1991;154:607-611.
Skowronski E, Hartman K. Obstetric management following traumatic tetraplegia: case series and literature review. Aust N Z J Obstet Gynaecol 2008;48:485-491.
Lambert DH, Deane RS, Mazuzan JE. Anesthesia and the control of blood pressure in patients with spinal cord injury. Anesth Analg 1982;61:344-348.
Eltorai IM, Wong DH, Lacerna M, Comarr, AE, Montroy R. Surgical aspects of autonomic dysreflexia. J Spinal Cord Med 1997;20:361-364.
Matthews JM, Wheeler GD, Burnham RS, Malone LA, Steadward RD. The effects of surface anaesthesia on the autonomic dysreflexia response during functional electrical stimulation. Spinal Cord 1997;35:647-651.
Kim JH, Rivas DA, Shenot PJ, Green B, Kennelly M, Erickson, JR, O’Leary M, Yoshimura N, Chancellor MB. Intravesical resiniferatoxin for refractory detrusor hyperreflexia: a multicenter, blinded, randomized, placebo-controlled trial. J Spinal Cord Med 2003;26:358-363.
Giannantoni A, Di Stasi SM, Stephen RL, Navarra P, Scivoletto G, Mearini E, Porena M. Intravesical capsaicin versus resiniferatoxin in patients with detrusor hyperreflexia: a prospective randomized study. J Urol 2002;167:1710-1714.
Igawa Y, Satoh T, Mizusawa H, Seki S, Kato H, Ishizuka O, Nishizawa O. The role of capsaicin-sensitive afferents in autonomic dysreflexia in patients with spinal cord injury. BJU Int 2003;91:637-641.
Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139:919-922.
Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000;164:692-697.
Chen SL, Bih LI, Huang YH, Tsai SJ, Lin TB, Kao YL. Effect of single botulinum toxin A injection to the external urethral sphincter for treating detrusor external sphincter dyssynergia in spinal cord injury. J Rehabil Med 2008;40:744-748.
Kuo HC. Satisfaction with urethral injection of botulinum toxin A for detrusor sphincter dyssynergia in patients with spinal cord lesion. Neurourol Urodyn 2008; 27: 793-796.
Chen SF, Kuo HC. Improvement in autonomic dysreflexia after detrusor onabotulinumtoxinA injections in patients with chronic spinal cord injuries. Tzu Chi Medical Journal 2012;24:201-204.
Giannantoni A, Di Stasi SM, Scivoletto G, Mollo A, Silecchia A, Fuoco U, Vespasiani G. Autonomic dysreflexia during urodynamics. Spinal Cord 1998;36:756-860.
Bycroft J, Shergill I, Choong E, Arya N, Shah P. Autonomic dysreflexia: a medical emergency. Postgraduate Medical Journal. 2005;81(954):232-235.
Image Credits
Author: SCIRE Community Team | Reviewer: Shannon Sproule | Published: 10 October 2017 | Updated: ~
Functional electrical stimulation is a treatment that activates muscles below the spinal cord injury (SCI) during exercise and activity. This page outlines basic information about functional electrical stimulation and its use for movement and strength after SCI.
Key Points
Functional electrical stimulation (FES) is a type of neuromodulation where electrical stimulation is applied to the nerves located outside the spinal cord and brain. This stimulation causes the muscles to contract and can assist with purposeful or functional movement in weak or paralyzed muscles.
FES is delivered using a variety of handheld or specialized commercial electrical therapy machines connected to electrodes that are placed on the skin’s surface. Systems are also available with implanted electrodes in the muscles, although this is very specialized and not widely available.
Muscle stimulation is used for several reasons after SCI:
To promote movement and strength in weak or paralyzed muscles: Muscle stimulation is used early in rehabilitation to promote movement in muscles that are not moving or only producing a flicker of movement. It may promote recovery of movement function by assisting with normal movements and with repetition of movements.
To improve fitness and health: When FES is used as part of a rhythmic exercise like cycling, walking, or rowing, it can help to maintain health of the heart, lungs, and circulation. It may also help to maintain healthy bones.
To assist with functional movement activities like stepping, getting up to standing, and grasping: FES can be used to assist with purposeful movements by improving muscle contractions (for weakened muscles), mobility or range of movement as well as possibly decreasing spasticity.
To maintain muscle mass below the SCI: Regular use of FES may help to prevent muscle loss that happens when the muscles that are paralyzed are not used. Unless neurological return occurs this improvement will stop if the FES is discontinued.
To control the muscles of breathing and bladder function: This includes the use of surgically implanted diaphragmatic pacers (FES systems that create muscle contractions in the diaphragm to stimulate regular breaths) and bladder control systems (FES systems that stimulate the muscles of urination). However, this page will focus on FES used for movement and strength after SCI.
Watch our YouTube video about FES!
You may hear other names for FES such as “neuromuscular electrical stimulation” (NMES) or simply “electrical stimulation” (ES). These terms are often used to refer to electrical stimulation of the muscles during more passive activities (like lying down or sitting), while ”FES” usually describes stimulation during purposeful activities like cycling or walking. However, in practice these terms are often used interchangeably to describe similar or related treatments and the goals of all are to promote strength, movement and function and decrease pain and spasticity.
There are a number of other neuromodulation techniques that are used for various purposes in SCI, including transcutaneous electrical nerve stimulation, sacral nerve stimulation, and intrathecal Baclofen, described in other SCIRE Community articles.
It is important to speak with a health provider about using FES to make sure it is safe and suitable for you and to learn how to use the equipment correctly.
FES is usually applied through electrodes that are placed on the surface of the skin, although electrodes can also be implanted into the muscles. Electrodes are placed over nerves or part of the muscles below the SCI that respond well to electrical stimulation. The electrodes are then attached to an adjustable machine that generates the stimulation. Your health provider will determine the settings that are used for the treatment and how long it will last.
The electrical stimulation is then gradually turned up until the muscles begin to tense or contract. Depending on your sensation, as the machine is turned up, you may feel pins and needles or other unusual sensations, which may take some time to get used to. The aim is to create a forceful but tolerable muscle contraction.
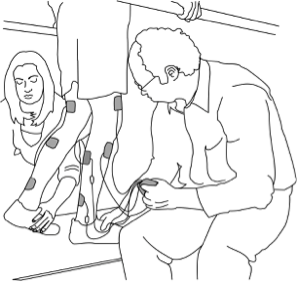
FES is applied through electrodes on the leg muscles during assisted walking.2
If the electrical stimulation goes well, it is then combined with a movement task. This may be as simple as lifting a wrist or ankle or more complex such as cycling on a stationary bike, rowing on a rowing machine, grasping, or stepping in parallel bars or a body weight support treadmill system.
The length of each session will vary depending on the goals of the treatment. Time may be required to enable your muscles to tolerate longer sessions as the muscles may fatigue quickly. Sessions are usually done several times per week for several weeks to gain training benefits.
Your health provider will monitor your response to the treatment and inspect the skin for any redness or irritation after the treatment has ended. Once you have learned to use FES safely, you may be able to use it on your own.
Our bodies naturally use electrical signals as part of the nervous system. When we move, the brain generates and sends electrical impulses along the spinal cord and nerves to tell the muscles to move.
Spinal cord injury can interrupt this pathway, preventing electrical impulses from passing through the spinal cord to reach the muscles. However, if the nerves and muscles below the injury are not damaged, they can still respond to electrical signals.

FES electrodes are placed over nerves or over electrically-sensitive parts of the muscles below the SCI. The specific type of electrical stimulation used with FES can trigger the nerve cells of movement (motor neurons) to send signals that cause muscle movement. An intact peripheral nerve and healthy muscle tissue are required to enable the external source of electricity to facilitate muscle contraction.
FES can only be used for muscle weakness or paralysis caused by injuries to the spinal cord, but not injuries to the conus medullaris, cauda equina, or the nerves outside of the spinal cord. The nerve cells in these structures (called lower motor neurons) must to be intact for FES to work.
Like exercise, regular treatment with FES is usually needed to maintain the effects of the treatment. For people with complete injuries, when FES treatments are stopped, the treatment effects will usually go away over time. For people with incomplete injuries, the goal is for some carryover of strength and movement be retained after the treatment is stopped.
 There are some situations in which FES may be unsafe to use. This not a complete list, speak to a health provider about your health history and whether FES is safe for you.
There are some situations in which FES may be unsafe to use. This not a complete list, speak to a health provider about your health history and whether FES is safe for you.
FES is often used with the following conditions after SCI but should be monitored closely. Speak to your health provider for more information.
FES is generally well tolerated by people who can use it safely (see above for when FES may be unsafe). Serious medical complications from FES are rare. However, there are risks and side effects that should be discussed with a health provider before using FES.
In some cases, risks and side effects may be caused by improper use of the equipment. It is essential to learn to use the equipment from a health provider and to only use FES according to their direction and with the settings that they recommend.
For some people, side effects of FES may be stronger at first, but as their body gets used to FES with repeated treatments, their physical reactions may reduce over time.
Several studies have shown that FES helps to improve strength and fitness after SCI.
 Studies have shown that both FES arm exercise and FES cycling helps to maintain or improve strength after SCI. However, FES cycling may be more effective for maintaining strength after injury than improving strength that has already been lost. This is supported by moderate evidence from five studies.
Studies have shown that both FES arm exercise and FES cycling helps to maintain or improve strength after SCI. However, FES cycling may be more effective for maintaining strength after injury than improving strength that has already been lost. This is supported by moderate evidence from five studies.
 Fifteen studies have looked at FES for improving many different aspects of fitness after SCI. Taken altogether, these studies provide weak evidence that FES training done at least 3 days per week for 2 months helps to improve many aspects of cardiovascular fitness after SCI.
Fifteen studies have looked at FES for improving many different aspects of fitness after SCI. Taken altogether, these studies provide weak evidence that FES training done at least 3 days per week for 2 months helps to improve many aspects of cardiovascular fitness after SCI.
 Studies show that FES improves walking speed and distance in people with both incomplete and complete SCI. Some of these studies also showed that regular use of FES carried over to improve walking even without FES. This is supported by weak evidence from eight studies.
Studies show that FES improves walking speed and distance in people with both incomplete and complete SCI. Some of these studies also showed that regular use of FES carried over to improve walking even without FES. This is supported by weak evidence from eight studies.
The effects of FES treatment may also help to prevent complications of SCI like pressure sores, bone loss, spasticity, and orthostatic hypotension. These benefits may accompany gains in strength or fitness related to FES treatment.
 Although it is commonly thought that increased muscle bulk from FES will reduce the risk of pressure sores, there are not very many studies which have looked at whether this actually happens. One study provides weak evidence that FES cycling for 2 years reduced the number of pressure ulcers that occurred after SCI. Another study showed that regular FES cycling showed a trend toward reducing seat pressures.
Although it is commonly thought that increased muscle bulk from FES will reduce the risk of pressure sores, there are not very many studies which have looked at whether this actually happens. One study provides weak evidence that FES cycling for 2 years reduced the number of pressure ulcers that occurred after SCI. Another study showed that regular FES cycling showed a trend toward reducing seat pressures.
 Research studies show that FES cycling does not prevent bone loss after SCI (moderate evidence from two studies). However, it may help to increase bone density that has already been lost, although the evidence for this is conflicting (based on six studies). It is not clear whether any gains in bone density last long-term or if continued FES treatment is needed for them to be maintained.
Research studies show that FES cycling does not prevent bone loss after SCI (moderate evidence from two studies). However, it may help to increase bone density that has already been lost, although the evidence for this is conflicting (based on six studies). It is not clear whether any gains in bone density last long-term or if continued FES treatment is needed for them to be maintained.
For more information on bone density, read our article about Osteoporosis.
It is not clear what effects FES has on spasticity after SCI. There is conflicting evidence from three studies about whether FES cycling can help to reduce spasticity after SCI.
Click here for our article on Spasticity.
Three studies provide moderate evidence that FES of the legs during a single change in position reduced orthostatic hypotension. However, this only shows that FES prevents orthostatic hypotension while it is applied, and further research is needed to look at what benefits this could have to people living with SCI.
Overall, the research evidence suggests that FES is most likely effective for improving muscle strength after SCI. It may also have effects on fitness, walking skills, bone density, skin health, spasticity, and orthostatic hypotension, although more high quality research is needed to confirm. FES appears to be safe when used appropriately and is widely available in most rehabilitation settings. Discuss this treatment with your health providers to find out if it is a suitable treatment option for you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Professional “Lower Limb”, “Upper Limb”, “Bone Health”, “Cardiovascular Health and Exercise”, “Orthostatic Hypotension”, “Pressure Ulcers”, and “Spasticity” chapters:
Lam T, Wolfe DL, Domingo A, Eng JJ, Sproule S (2014). Lower Limb Rehabilitation Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-74.
Available from: http://scireproject.com/evidence/lower-limb/
Connolly SJ, McIntyre A, Mehta, S, Foulon BL, Teasell RW. (2014). Upper Limb Rehabilitation Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0: p 1-77.
Available from: http://scireproject.com/evidence/upper-limb/
Craven C, Lynch CL, Eng JJ (2014). Bone Health Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 37.
Available from: https://scireproject.com/evidence/bone-health/
Warburton DER, Krassioukov A, Sproule S, Eng JJ (2014). Cardiovascular Health and Exercise Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p. 1-48.
Available from: https://scireproject.com/evidence/cardiovascular-health-and-exercise/
Krassioukov A, Wecht JM, Teasell RW, Eng JJ (2014). Orthostatic Hypotension Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p. 1-26.
Available from: https://scireproject.com/evidence/orthostatic-hypotension/
Hsieh J, McIntyre A, Wolfe D, Lala D, Titus L, Campbell K, Teasell R. (2014). Pressure Ulcers Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. 1-90.
Available from: https://scireproject.com/evidence/skin-integrity-and-pressure-injuries/
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Curt A, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0.
Available from: https://scireproject.com/evidence/spasticity/
Evidence for “Strength” is based on the following studies:
[1] Baldi JC, Jackson RD, Moraille R, and Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 1998;36:463-469.
[2] Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C, and Scremin OU. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil 1999;80:1531-1536.
[3] Crameri RM, Weston A, Climstein M, Davis GM, and Sutton JR. Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sports 2002;12:316-322.
[4] Gerrits HL, de Haan A, Sargeant AJ, Dallmeijer A, and Hopman MT. Altered contractile properties of the quadriceps muscle in people with spinal cord injury following functional electrical stimulated cycle training. Spinal Cord 2000;38:214-223.
[5] Needham-Shropshire BM, Broton JG, Cameron TL, Klose J. Improved motor function in tetraplegics following neuromuscular stimulation-assisted arm ergometry. J Spinal Cord Med 1997;20:49-55.
[6] Cameron T, Broton JG, Needham-Shropshire B, Klose KJ. An upper body exercise system incorporating resistive exercise and neuromuscular electrical stimulation (nms). J Spinal Cord Med 1998;21:1-6.
Evidence for “Cardiovascular Fitness” based on:
[1] Berry HR, Kakebeeke TH, Donaldson N, Perret C, Hunt KJ. Energetics of paraplegic cycling: adaptation to 12 months of high volume training. Technology and Health Care 2012; 20: 73-84.
[2] Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614-22.
[3] Zbogar D, Eng JJ, Krassioukov AV, Scott JM, Esch BT, Warburton DE. The effects of functional electrical stimulation leg cycle ergometry training on arterial compliance in individuals with spinal cord injury. Spinal Cord 2008;46(11):722-6.
[4] Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve 2004;29(1):104-11.
[5] Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol 1997;273(3 Pt 2):R1072-9.
[6] Mohr T, Andersen JL, Biering-Sorensen F, Galbo H, Bangsbo J, Wagner A, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord 1997;35(1):1-16.
[7] Barstow TJ, Scremin AM, Mutton DL, Kunkel CF, Cagle TG, Whipp BJ. Changes in gas exchange kinetics with training in patients with spinal cord injury. Med Sci Sports Exerc 1996;28(10):1221-8.
[8] Faghri PD, Glaser RM, Figoni SF. Functional electrical stimulation leg cycle ergometer exercise: training effects on cardiorespiratory responses of spinal cord injured subjects at rest and during submaximal exercise. Arch Phys Med Rehabil 1992;73(11):1085-93.
[9] Hooker SP, Figoni SF, Rodgers MM, Glaser RM, Mathews T, Suryaprasad AG, et al. Physiologic effects of electrical stimulation leg cycle exercise training in spinal cord injured persons. Arch Phys Med Rehabil 1992;73(5):470-6.
[10] Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil 2001;82(6):832-9.
[11] Ragnarsson KT, Pollack S, O’Daniel W, Jr., Edgar R, Petrofsky J, Nash MS. Clinical evaluation of computerized functional electrical stimulation after spinal cord injury: a multicenter pilot study. Arch Phys Med Rehabil 1988;69(9):672-7.
[12] Taylor JA, Picard G, Widrick JJ. Aerobic capacity with hybrid FES rowing in spinal cord injury: comparison with arms-only exercise and preliminary findings with regular training. PM R 2011;3(9):817-24.
[13] Kahn NN, Feldman SP, Bauman WA. Lower-extremity functional electrical stimulation decreases platelet aggregation and blood coagulation in persons with chronic spinal cord injury: a pilot study. J Spinal Cord Med 2010;33(2): 150-8.
[14] Hakansson NA, Hull ML. Can the efficacy of electrically stimulating pedaling using a commercially available ergometer be improved by minimizing the muscle stress-time integral? Muscle Nerve 2012; 45:393-402.
Evidence for “Walking” is based on the following studies:
[1] Thrasher TA, Flett HM, and Popovic MR. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord 2006;44:357-361.
[2] Ladouceur M, and Barbeau H. Functional electrical stimulation-assisted walking for persons with incomplete spinal injuries: changes in the kinematics and physiological cost of overground walking. Scand J Rehabil Med 2000a;32:72-79.
[3] Ladouceur M, and Barbeau H. Functional electrical stimulation-assisted walking for persons with incomplete spinal injuries: longitudinal changes in maximal overground walking speed. Scand J Rehabil Med 2000b;32:28-36.
[4] Wieler M, Stein RB, Ladouceur M, Whittaker M, Smith AW, Naaman S, Barbeau H, Bugaresti J, and Aimone E. Multicenter evaluation of electrical stimulation systems for walking. Arch Phys Med Rehabil 1999;80:495-500.
[5] Klose KJ, Jacobs PL, Broton JG, Guest RS, Needham-Shropshire BM, Lebwohl N, Nash MS, and Green BA. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 1. Ambulation performance and anthropometric measures. Arch Phys Med Rehabil 1997;78:789-793.
[6] Granat MH, Ferguson AC, Andrews BJ, and Delargy M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury–observed benefits during gait studies. Paraplegia 1993;31:207-215.
[7] Stein RB, Belanger M, Wheeler G, Wieler M, Popovic DB, Prochazka A, and Davis LA. Electrical systems for improving locomotion after incomplete spinal cord injury: an assessment. Arch Phys Med Rehabil 1993;74:954-959.
[8] Granat M, Keating JF, Smith AC, Delargy M, and Andrews BJ. The use of functional electrical stimulation to assist gait in patients with incomplete spinal cord injury. Disabil Rehabil 1992;14:93-97.
Evidence for “Bone health” is based on the following studies:
[1] Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stussi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest 2003;33:412-419.
[2] Lai CH, Chang WHS, Chan WP, Peng CW, Shen LK, Chen JJJ, Chen SC. Effects of Functional Electrical Stimulation Cycling Exercise on Bone Mineral Density Loss in the Early Stages of Spinal Cord Injury. J Rehabil Med 2010; 42:150-154.
[3] Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int 1997;61:22-25.
[4] Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW, Chen JJ. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil 2005;27:1337-1341.
[5] Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson Nde N, Eser P. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008 Jul;43(1):169-76. Epub 2008 Mar 20.
[6] Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J. Muscle and bone in paraplegic patients, and the effect of functional electrical stimulation. Clin Sci (Lond) 1988;75:481-487.
[7] Leeds EM, Klose J, Ganz W, Serafini A, Green BA. Bone mineral density after bicycle ergometry training. Archives of Physical Medicine and Rehabilitation 1990;71:207-9.
[8] BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord-injured patients. Am J Phys Med Rehabil 1996;75:29-34.
Evidence for “Pressure Ulcers” is based on the following studies:
[1] Dolbow DR, Gorgey AS, Dolbow JD, Gater DR. Seat pressure changes after eight weeks of functional electrical stimulation cycling: a pilot study. Top Spinal Cord Inj Rehabil. 2013 Summer;19(3):222-8.
[2] Petrofsky JS. Functional electrical stimulation, a two year study. J Rehabil. 1992;58(3):29–34
Evidence for “Spasticity” is based on the following studies:
[1] Kapadia N, Masani K, Craven B, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med 2014;37:511-24.
[2] Manella K & Field-Fote E. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci 2013;31:633-46.
[3] Ralston K, Harvey L, Batty J, et al. Functional electrical stimulation cycling has no clear effect on urine output, lower limb swelling, and spasticity in people with spinal cord injury: A randomised cross-over trial. J Physiother 2013;59:237-43.
[4] Kuhn D, Leichtfried V, Schobersberger W. Four weeks of functional electrical stimulated cycling after spinal cord injury: a clinical cohort study. Inter J Rehabil Res 2014;37:243-50.
[5] Mazzoleni S, Stampacchia G, Gerini A, Tombini T, Carrozza M. FES-cycling training in spinal cord injured patients. Eng Med Biol Soc 2013:5339-41.
[6] Sadowsky C, Hammond E, Strohl A, et al. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med 2013;36:623-31.
[7] Reichenfelser W, Hackl H, Hufgard J, Kastner J, Gstaltner K, Gföhler M. Monitoring of spasticity and functional ability in individuals with incomplete spinal cord injury with a functional electrical stimulation cycling system. J Rehabil Med 2012;44:444-9.
[8] Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil 2008;22:627-34.
[9] Mirbagheri M, Ladouceur M, Barbeau H, Kearney R. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured
[10] Granat M, Ferguson A, Andrews B, Delargy M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury–observed benefits during gait studies. Paraplegia 1993;31:207-15.
[11] Thoumie P, Le C, Beillot J, Dassonville J, Chevalier T, Perrouin-Verbe B et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia 1995;33:654-9.
Evidence for “Orthostatic Hypotension” is based on the following studies:
[1] Faghri PD, Yount J. Electrically induced and voluntary activation of physiologic muscle pump: a comparison between spinal cord-injured and able-bodied individuals. Clin Rehabil 2002;16:878-885.
[2] Elokda AS, Nielsen DH, Shields RK. Effect of functional neuromuscular stimulation on postural related orthostatic stress in individuals with acute spinal cord injury. J Rehabil Res Dev 2000;37:535-542.
[3] Sampson EE, Burnham RS, Andrews BJ. Functional electrical stimulation effect on orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil 2000; 81: 139-143.
Other references:
Electrophysical Agents – Contraindications And Precautions: An Evidence-Based Approach To Clinical Decision Making In Physical Therapy. Physiother Can. 2010 Fall;62(5):1-80.
Gibbons RS, Shave RE, Gall A, Andrews BJ. FES-rowing in tetraplegia: a preliminary report. Spinal Cord. 2014 Dec;52(12):880-6.
Martin R, Sadowsky C, Obst K, Meyer B, McDonald J. Functional electrical stimulation in spinal cord injury: from theory to practice. Top Spinal Cord Inj Rehabil. 2012 Winter;18(1):28-33.
Warms CA, Backus D, Rajan S, Bombardier CH, Schomer KG, Burns SP. Adverse events in cardiovascular-related training programs in people with spinal cord injury: a systematic review. J Spinal Cord Med. 2014 Nov;37(6):672-92.