Authors: SCIRE Community Team | Reviewer: Andrea Townson and Janice Eng | Published: 2 April 2019 | Updated: Apr 20, 2020
Cannabis (marijuana) is an alternative treatment option for pain and spasticity after spinal cord injury (SCI). This page outlines basic information about cannabis and its use after SCI.
Key Points
- “Cannabis” refers to products derived from the cannabis plant, such as marijuana. The natural cannabinoids or compounds found in cannabis can also be made synthetically.
- Cannabis may be inhaled as a smoke or vapour or taken by mouth as a capsule or spray.
- Smoking cannabis is not recommended due to the risks associated with inhaling smoke.
- The safety of cannabis products for use after SCI is not known. Please consult your health providers for detailed safety information.
- Research on cannabis use after SCI is in its early stages. Studies done so far show that cannabis products may have beneficial effects on pain and are unclear about its effects on spasticity. More research is needed to establish if cannabis is a safe and effective treatment after SCI.
Cannabis is a term that refers to the products of cannabis (hemp) plants, a group of plants from central Asia that are now cultivated around the world. Cannabis sativa, Cannabis indica, and Cannabis ruderalis are three well-known types of cannabis, but many strains or varieties exist, both pure and hybrid types. Common preparations of cannabis include marijuana, which is the dried leaves and flowering tops of the plant, and hashish, which is its condensed resin. Cannabis has been used for thousands of years as a medicine and recreational drug.
Currently, cannabis is a controlled substance in most regions because of its psychoactive effects. However, exceptions are made in some places for approved medical or spiritual uses. In addition to medical use, in Canada recreational use of cannabis has also been made legal as of October 2018. Here, the sale of recreational cannabis was originally limited to dried cannabis and oils, but as of October 2019 edibles and concentrates are also legal for sale.
Cannabis has been studied as a treatment for conditions as diverse as nausea associated with cancer chemotherapy, loss of appetite in people with HIV, and spasticity associated with multiple sclerosis.
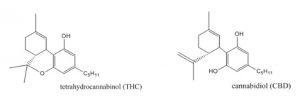
Cannabis has its unique properties because of naturally-occurring chemical compounds within the plant called cannabinoids. Cannabinoids act on receptors on the surface of cells called cannabinoid receptors, causing effects on body processes like pain, memory, appetite, and immune responses.
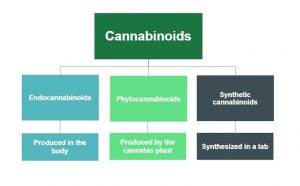
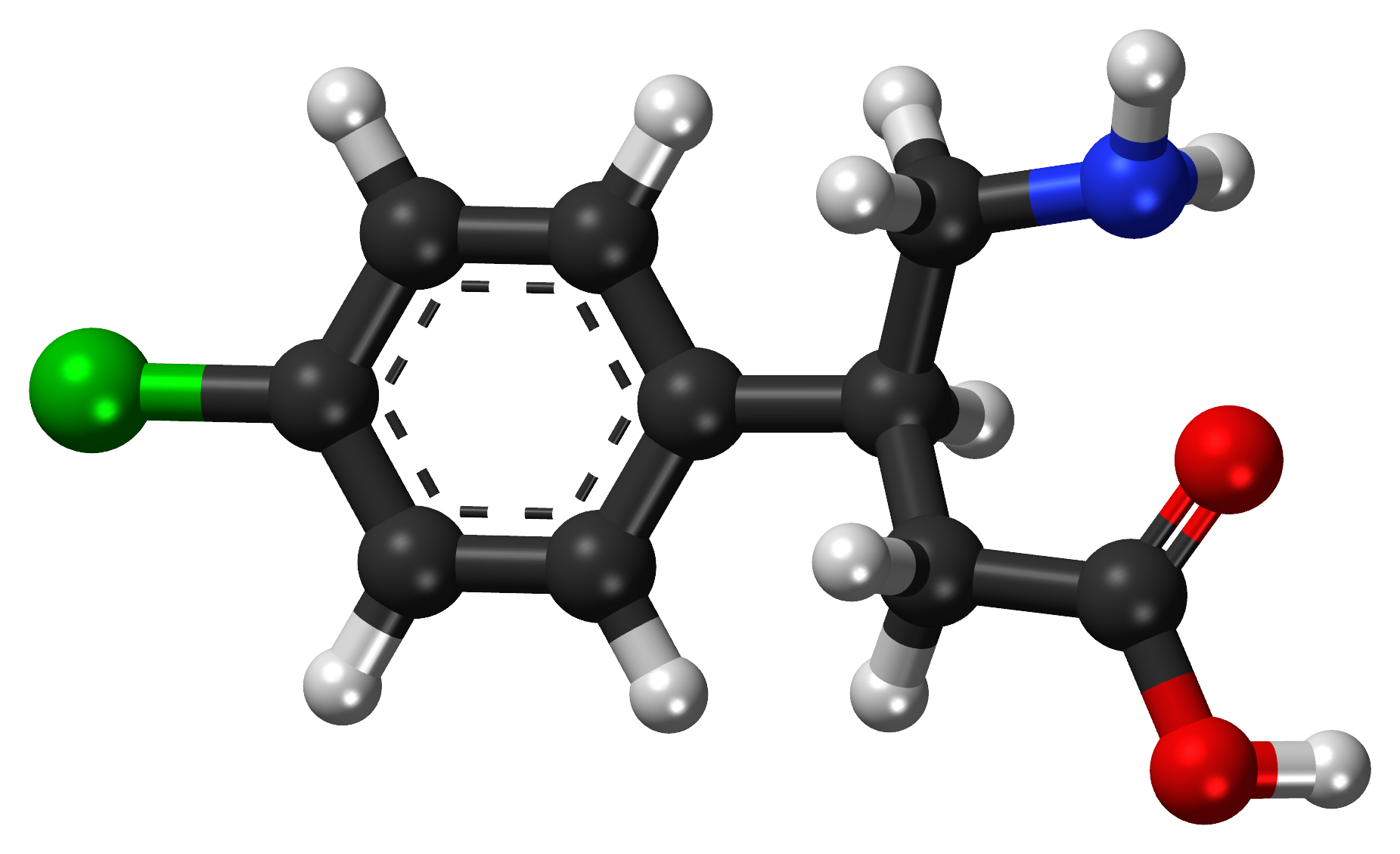
Cannabinoids occur naturally within the body (endocannabinoids), in cannabis plants (phytocannabinoids), and can also be synthesized in a lab (synthetic cannabinoids). There are more than 60 cannabinoids present in cannabis, with the most well-known being Delta-9-tetrahydrocannabinol (commonly known as THC), which is responsible for many of the psychoactive effects for which cannabis is known such as creating a “high” or sense of euphoria. Other cannabinoids, like Cannabidiol (also known as CBD), are not psychoactive and may have different effects such as improving mental health concerns and preventing oxidative damage although evidence for this is currently not conclusive. Because of these benefits over THC as well as the reduced health risks, CBD is believed to be the component of cannabis that gives rise to its medicinal potential and opposes the negative psychiatric effects associated with THC.
Cannabis/Cannabinoids, whether plant-derived or human-made, may be used for medicinal or recreational purposes in a variety of ways.
Medical cannabinoid products
Medical cannabis
The laws and regulations required to get approval for medical marijuana differ by country and region. In Canada, use of medical cannabis requires authorization for use from a physician.
Prescription synthetic cannabinoids
In some countries, certain synthetic cannabinoids are available for therapeutic use and require a prescription from a physician. Like other medications, these products are registered with a Drug Identification Number (DIN) in Canada or with the Food and Drug Administration (FDA) in the United States. Prescription synthetic cannabinoids are carefully regulated and monitored for their composition and effects on the body and are developed to minimize accompanying intoxication.
Recreational cannabis products

There are various environmental and health risks associated with unlicensed grow-ops.4
Recreational use of cannabis is legal in Canada, but still subject to provincial or territorial restrictions. Recreational use outside these restrictions is illegal. Like medical cannabis, the production and distribution of recreational cannabis is regulated to ensure safety and quality. There are various concerns with the use of cannabis that is not regulated or produced legally. These cannabis products may include harmful contaminants (e.g., mold, bacteria, and pesticides) or have much greater variation in their chemical composition than cannabis products intended for medical use. It can be difficult to know exactly what dose you are receiving and the risks and side effects for using these products may be unknown. Another issue with cannabis sourced from illegal grow-ops include its negative impact on the environment as these sites may misuse toxic pesticides and may divert water supply away from lakes or rivers, threatening plant, wildlife, and human health. Unregulated cannabis products are not recommended for treating symptoms of SCI.
Illegal synthetic cannabinoids

Illegal synthetic cannabinoids may be sold to look like cannabis.5
Even though synthetic cannabinoids act on the same receptors as the phytocannabinoids found in the cannabis plant, they may produce different effects on the body. Some non-prescription synthetic cannabinoids are made to imitate the psychoactive effects of THC, making them potentially dangerous especially since their actions on the body can be unpredictable. Known by names like “Spice” and “K2,” these compounds are often combined with plant-based products and sold as “alternatives” to marijuana. However, all activities associated with non-prescription synthetic cannabinoids (e.g., production, distribution, use) are illegal in Canada. Besides the fact that illegal synthetic cannabinoids have not been tested in humans, their product composition can vary greatly and may be laced with other unknown and potentially deadly substances. Synthetic cannabinoids also more potent than plant-derived THC. This means that they bind more strongly to the cannabinoid receptors, increasing the risk of overdose.
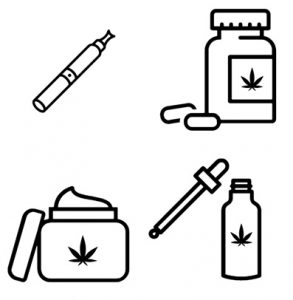
Cannabis products are usually inhaled or taken by mouth. Smoking is the most common method among the general population as well as within the SCI population. However, there are serious concerns about the negative health effects to the user and those nearby associated with inhaling and exhaling smoke, which contains many of the same harmful compounds as tobacco smoke. People with SCI, in particular, should avoid smoking cannabis as respiratory issues including compromised breathing and pneumonia are already prevalent in the SCI population. Vaporization is another method where the cannabis leaves are heated to form a vapour that is then inhaled. While vaping prevents the cannabinoids from burning which decreases the amount of toxic by-products produced compared to smoking, it is not without risks and has recently been associated with vaping-associated pulmonary injury (VAPI). After a sharp increase in VAPI cases in August and September of 2019, emergency department visits continue to decline. This is thought to be due to the removal of vitamin E acetate from most products, increased public awareness of the risks associated with THC containing e-cigarettes or vaping devices, and law enforcement actions related to illicit products in the US. Canadian extracts for vaping that contain THC are not allowed to have any added vitamins, minerals, nicotine, sugars, flavouring or colouring agents.
Cannabis can also be taken by mouth in the form of food items or other products like oils, capsules, and mouth sprays. Other less common methods cannabis may be delivered include through the skin (e.g. creams, lotions, balms, patches, etc.), through the rectum, or into the veins
Cannabidiol oil
CBD oil is becoming more popular among people who wish to gain the health benefits of cannabis and avoid the psychoactive effects of THC. Although many people use CBD oil for a range of ailments, there is limited safety and efficacy data (and no research in SCI) to support its use for these conditions. Recently, positive results from three clinical trials with strong evidence have led the Food and Drug Administration (FDA) in the United States to approve the use of CBD oil for two rare forms of epilepsy in June 2018.
Prescription synthetic cannabinoids
Prescription synthetic cannabinoids often use isolated cannabinoid compounds or combinations of cannabinoids. This includes products such as:
- Nabilone (Cesamet), a synthetic cannabinoid similar to THC that is taken by mouth as a capsule.
- Dronabinol (Marinol), synthetic THC that is taken by mouth as a capsule. Please note that dronabinol is no longer available in Canada.
- Nabiximols (Sativex), a mix of cannabis plant-derived THC and CBD that is taken as a mouth spray.
There are currently no standard cannabis dosing regimens for SCI-related conditions. Dosing for medical cannabis varies based on factors such as method of delivery, past cannabis use, and the medical condition being treated. Additionally, the amount of THC and CBD in marijuana is not always the same. Thus, the effects of different marijuana products are not always the same. Levels of THC and CBD in a product can change based on the strain of the plant used as well as how the plant was grown and prepared.
Especially for those who have never used cannabis in the past, it is recommended that they start on low doses before slowly increasing the dose until their therapeutic goals are met. To minimize negative side effects related to THC and maximize symptom control, a strain with low THC and high CBD may be used initially. Immediately discontinue use if any intolerable side effects occur.
People who use cannabis for medicinal purposes consume an average of 1-3 g/day or 10-20 g/week. Even with equal grams of the same cannabis strain, the amount of cannabis the body actually absorbs differs depending on the method of delivery. For example, people who wish to switch from inhaling cannabis to taking cannabis by mouth may need to increase in their daily cannabis use by 2.5 times to get an equivalent dose. Each different form and method of cannabis use will change how quickly the drug produces an effect and how long it lasts in the body. For example, inhalation of cannabis will generally lead to a faster onset of action and longer-lasting effect than oral ingestion.
| Inhalation | Oral ingestion | |
| Onset of action | Few minutes | 30 minutes (up to 3-4 hours) |
| Peak of effect | 30 minutes | 3-4 hours |
| Duration of effect | 2-4 hours (up to 24 hours) | 8 hours (up to 12-24 hours) |
It is important that you closely follow the directions of your health providers and consult with them before making any changes to your cannabis use. Speak to your health provider for more detailed information.
The safety of medical cannabis use after SCI is not yet known. However, a number of risks and side effects of cannabis use in the general population are known. Many of the short-term side effects of cannabis have been reported to be mild to moderately severe and related to the dose of the drug taken. Uncommon but serious adverse effects may also exist. Furthermore, the risks to long-term users are not well known and some side effects may be related to regular use over time.
This is not a complete list. Speak to your health provider for detailed information about the risks and side effects of cannabis use.
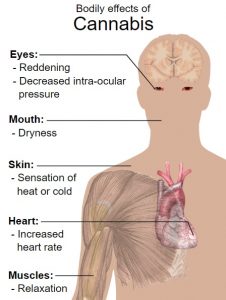
Short-term side effects of cannabis may include:
- Dizziness and lightheadedness
- Dry mouth, throat irritation, and cough
- Drowsiness
- Altered judgment and attention
- Anxiety and agitation
- Hallucinations
- Disorientation and confusion
- Increased heart rate
- Impaired coordination and balance
- Impaired short-term memory
- Headache
- Paranoia and psychosis
- Reddening of the eyes
- Decreased intra-ocular pressure (pressure within the eyes)
- Muscle relaxation
- Interactions with other medications
Because cannabis lingers in the body long after use, task performance may be impaired for up to 24 hours. It is recommended to avoid operating heavy machinery or performing dangerous activities for 3-4 hours after inhaling cannabis, 6 hours after oral ingestion of cannabis, and 8 hours if a “high” is experienced. Examples of high-risk activities may include performing transfers and participating in physical therapy sessions.
Long-term cannabis use may be associated with:
- Addiction and withdrawal
- Airway problems like chronic bronchitis
- Possible increased risk of mental disorders like anxiety, depression, schizophrenia, and psychosis in people at risk for these conditions
- Possible increased cancer risk with long term smoking, although this is not yet clear
An emerging concern is the effects that cannabis use may have on adolescents and young adults. Studies have suggested that cannabis use early in adolescence may alter brain development and could be related to the development of psychotic disorders as adults.

Studies show that cannabis is mostly used by patients with SCI for (chronic) pain and spasm relief, as well as for anxiety, stress and depression, bowel and bladder management, nausea, to increase appetite, to improve sleep, to decrease other medication use and for pleasure, recreation and relaxation. However, research has only studied the use of cannabinoid products in the treatment of pain and spasticity after SCI.
Pain
Early research provides moderate evidence that smoked and vapourized cannabis may help to reduce neuropathic pain. There is also weak evidence that oral plant-derived cannabinoid sprays may help to reduce neuropathic pain. Moderate evidence from two other studies indicates no benefit with synthetic cannabinoids. In one, dronabinol was no different than diphenhydramine (an anti-allergy medication with no pain-relieving properties) for reducing neuropathic pain. In the other, a synthetic cannabinoid called Normast showed no benefit. These last two studies were specific to people with SCI, while the other studies above also included people with other neurological conditions. Further research specific to people with SCI is needed to determine if cannabis and synthetic cannabinoids are safe and effective for pain after SCI.
Hear Matt describe his experience with synthetic and non-synthetic marijuana for pain management.
Spasticity
Research on cannabinoid products for spasticity after SCI has been conflicting. Four studies provide moderate evidence that synthetic cannabinoids and vapourized cannabis may help with spasticity after SCI. However, two other studies with moderate evidence have been inconclusive about whether cannabinoid products helped.
Overall, these studies show that cannabinoid-based treatments may have benefits in the treatment of spasticity, but further research through larger and more rigorous studies are needed before conclusions can be drawn about how effective they are.
There is early evidence that cannabinoid products may help to treat neuropathic pain after SCI and conflicting evidence about whether they help to treat spasticity after SCI. More studies are needed to confirm these findings.
It is not known whether cannabis is safe to use after SCI, especially over the long term, since cannabis use is associated with a number of potential risks and side effects. Until more research is done, it is important that you discuss this treatment option with your health providers in detail to find out if it is a suitable and safe treatment option for you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Mehta S, Teasell RW, Loh E, Short C, Wolfe DL, Hsieh JTC (2014). Pain Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0: p 1-79.
Available from: https://scireproject.com/evidence/rehabilitation-evidence/pain-management/
Hsieh JTC, Wolfe DL, Townson AF, Short C, Connolly SJ, Mehta S, Curt A, Foulon BL (2012). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan V, Mehta S, Sakakibara BM, Boily K, editors. Spinal Cord Injury Rehabilitation Evidence. Version 4.0.
Available from: https://scireproject.com/evidence/rehabilitation-evidence/spasticity/
Evidence for “What is cannabis” is based on:
Atakan, Z. (2012). Cannabis, a complex plant: different compounds and different effects on individuals. Therapeutic Advances in Psychopharmacology, 2(6), 241–254. https://doi.org/10.1177/2045125312457586
Baker, D., Pryce, G., Croxford, J. L., Brown, P., Pertwee, R. G., Huffman, J. W., & Layward, L. (2000). Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature, 404(6773), 84–87. https://doi.org/10.1038/35003583
Ben Amar, M. (2006). Cannabinoids in medicine: A review of their therapeutic potential. Journal of Ethnopharmacology, 105(1–2), 1–25. https://doi.org/10.1016/j.jep.2006.02.001
Birdsall, S. M., Birdsall, T. C., & Tims, L. A. (2016). The Use of Medical Marijuana in Cancer. Current Oncology Reports, 18(7), 40. https://doi.org/10.1007/s11912-016-0530-0
Evidence for “What are cannabinoids?” is based on:
Aizpurua-Olaizola, O., Elezgarai, I., Rico-Barrio, I., Zarandona, I., Etxebarria, N., & Usobiaga, A. (2017). Targeting the endocannabinoid system: future therapeutic strategies. Drug Discovery Today, 22(1), 105–110. https://doi.org/10.1016/j.drudis.2016.08.005
Zerrin 2012
Crippa, J. A., Guimarães, F. S., Campos, A. C., & Zuardi, A. W. (2018). Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Frontiers in Immunology, 9, 2009. https://doi.org/10.3389/fimmu.2018.02009
National Academies of Sciences, Engineering, and Medicine. 2017. The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. Washington, DC: The National Academies Press. doi: 10.17226/24625.
Whiting et al. (2015) Cannabinoids for Medical Use. A Systematic Review and Meta-Analysis. JAMA 313(24): 2456-2473.
Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W.(2018) Cannabis-based Medicine for chronic neurophathic pain in adults. Cochrane Database of Systematic Reviews, Issue 3. Art. No: CD012182 DOI: 10.1002/14651858.CD012182.pub2
Evidence for “How are cannabinoids used?” is based on:
Drossel, C., Forchheimer, M., & Meade, M. A. (2016). Characteristics of Individuals with Spinal Cord Injury Who Use Cannabis for Therapeutic Purposes. Topics in Spinal Cord Injury Rehabilitation, 22(1), 3–12. https://doi.org/10.1310/sci2201-3
Sheel, A. W., Welch, J. F., & Townson, A. (n.d.). Respiratory Management Following Spinal Cord Injury. Retrieved from www.scireproject.com
Health Canada (2018) Information for health care professionals. Cannabis (marihuana, marijuana) and the cannabinoids. Ottawa; Health Canada publications.
Center for Disease Control (2020) Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Retrieved on 13-02-2020 from: https://web.archive.org/web/20200213002533/https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
Evidence for “Cannabidiol oil” is based on:
Devinsky, O., Cross, J. H., Laux, L., Marsh, E., Miller, I., Nabbout, R., … Wright, S. (2017). Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. New England Journal of Medicine, 376(21), 2011–2020. https://doi.org/10.1056/NEJMoa1611618
Devinsky, O., Patel, A. D., Cross, J. H., Villanueva, V., Wirrell, E. C., Privitera, M., … Zuberi, S. M. (2018). Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. New England Journal of Medicine, 378(20), 1888–1897. https://doi.org/10.1056/NEJMoa1714631
Thiele, E. A., Marsh, E. D., French, J. A., Mazurkiewicz-Beldzinska, M., Benbadis, S. R., Joshi, C., … Wilfong, A. (2018). Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet, 391(10125), 1085–1096. https://doi.org/10.1016/S0140-6736(18)30136-3
Shannon, S., & Opila-Lehman, J. (2016). Effectiveness of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report. The Permanente Journal, 20(4), 16-005. https://doi.org/10.7812/TPP/16-005
Evidence for “What is the suggested dosing of cannabis?” is based on:
Health Canada. (2013). Information for Health Care Professionals Cannabis (marihuana, marijuana) and the cannabinoids. Retrieved from https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/marihuana/med/infoprof-eng.pdf
Evidence for “What are the risks and side effets of cannabis? Is based on:
Grant, I., Atkinson, J. H., Gouaux, B., & Wilsey, B. (2012). Medical marijuana: clearing away the smoke. The Open Neurology Journal, 6, 18–25. https://doi.org/10.2174/1874205X01206010018
Volkow, N. D., Baler, R. D., Compton, W. M., & Weiss, S. R. B. (2014). Adverse health effects of marijuana use. The New England Journal of Medicine, 370(23), 2219–2227. https://doi.org/10.1056/NEJMra1402309
Zhang, M. W., & Ho, R. C. M. (2015). The Cannabis Dilemma: A Review of Its Associated Risks and Clinical Efficacy. Journal of Addiction, 2015, 1–6. https://doi.org/10.1155/2015/707596
Health Canada. (2013). Information for Health Care Professionals Cannabis (marihuana, marijuana) and the cannabinoids. Retrieved from https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/marihuana/med/infoprof-eng.pdf
Evidence for “What are cannabinoids used for after spinal cord injury?” is based on:
Cardenas DD, Jensen MP. (2006) Treatments for chronic pain in persons with spinal cord injury: A survey study. The journal of spinal cord medicine 29:109-117.
Shroff FM. (2015) Experiences with Holistic Health Practices among Adults with Spinal Cord Injury. Rehabilitation Process and Outcome 4:27-34.
Drossel C, Forchheimer M, Meade MA. (2016) Characteristics of Individuals with Spinal Cord Injury Who Use Cannabis for Therapeutic Purposes. Top Spinal Cord Inj Rehabil;22:3-12.
Government of Canada (2019) Final regulations: Edible cannabis, cannabis extracts, cannabis topicals. Retrieved on 13-02-2020 from: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/resources/regulations-edible-cannabis-extracts-topicals.html
Andresen SR, Biering-Sorensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB. (2017) Cannabis use in persons with traumatic spinal cord injury in Denmark. J Rehabil Med 49:152-160.
Bruce D, Brady JP, Foster E, Shattell M. (2018) Preferences for Medical Marijuana over Prescription Medications Among Persons Living with Chronic Conditions: Alternative, Complementary, and Tapering Uses. Journal of alternative and complementary medicine (New York, NY) 24:146-153.
Hawley LA, Ketchum JM, Morey C, Collins K, Charlifue S. (2018) Cannabis Use in Individuals With Spinal Cord Injury or Moderate to Severe Traumatic Brain Injury in Colorado. Archives of physical medicine and rehabilitation 99:1584-1590.
Evidence for “Pain” is based on:
[1] Wilsey, B., Marcotte, T., Tsodikov, A., Millman, J., Bentley, H., Gouaux, B., & Fishman, S. (2008). A Randomized, Placebo-Controlled, Crossover Trial of Cannabis Cigarettes in Neuropathic Pain. The Journal of Pain, 9(6), 506–521. https://doi.org/10.1016/j.jpain.2007.12.010
[2] Wilsey, B., Marcotte, T. D., Deutsch, R., Zhao, H., Prasad, H., & Phan, A. (2016). An Exploratory Human Laboratory Experiment Evaluating Vaporized Cannabis in the Treatment of Neuropathic Pain From Spinal Cord Injury and Disease. The Journal of Pain, 17(9), 982–1000. https://doi.org/10.1016/j.jpain.2016.05.010
[3] Wade, D. T., Robson, P., House, H., Makela, P., & Aram, J. (2003). A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clinical Rehabilitation, 17(1), 21–29. https://doi.org/10.1191/0269215503cr581oa
[4] Rintala, D. H., Fiess, R. N., Tan, G., Holmes, S. A., & Bruel, B. M. (2010). Effect of Dronabinol on Central Neuropathic Pain After Spinal Cord Injury. American Journal of Physical Medicine & Rehabilitation, 89(10), 840–848. https://doi.org/10.1097/PHM.0b013e3181f1c4ec
Andresen, S.R., Bing, J., Hansen, R.M., Biering-Sørenson, F., Hagen, E.M., Rice, A.S., Nielsen, J.F., Bach, F.W., Finnerup, N.B., (2016) Ultramicronized palmitoylethanolamide in Spinal Cord Injury Neuropathic Pain: A Randomized, Double-blind, Placebo-controlled Trial. Pain. 157(9): 2097-103.
Evidence for “Spasticity” is based on:
[1] Pooyania, S., Ethans, K., Szturm, T., Casey, A., & Perry, D. (2010). A Randomized, Double-Blinded, Crossover Pilot Study Assessing the Effect of Nabilone on Spasticity in Persons With Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation, 91(5), 703–707. https://doi.org/10.1016/j.apmr.2009.12.025
[3] Maurer, M., Henn, V., Dittrich, A., & Hofmann, A. (1990). Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. European Archives of Psychiatry and Clinical Neuroscience, 240(1), 1–4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2175265
[4] Hagenbach, U., Luz, S., Ghafoor, N., Berger, J. M., Grotenhermen, F., Brenneisen, R., & Mäder, M. (2007). The treatment of spasticity with Δ9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord, 45(8), 551–562. https://doi.org/10.1038/sj.sc.3101982
[6] Grao-Castellote, C., Torralba-Collados, F., Gonzalez, L. M., & Giner-Pascual, M. (2017). [Delta-9-tetrahydrocannabinol-cannabidiol in the treatment of spasticity in chronic spinal cord injury: a clinical experience]. Revista de Neurologia, 65(7), 295–302. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28929471
[2] Wilsey, B., Marcotte, T. D., Deutsch, R., Zhao, H., Prasad, H., & Phan, A. (2016). An Exploratory Human Laboratory Experiment Evaluating Vaporized Cannabis in the Treatment of Neuropathic Pain From Spinal Cord Injury and Disease. The Journal of Pain, 17(9), 982–1000. https://doi.org/10.1016/j.jpain.2016.05.010
[5] Kogel, R. W., Johnson, P. B., Chintam, R., Robinson, C. J., & Nemchausky, B. A. (1995). Treatment of Spasticity in Spinal Cord Injury with Dronabinol, a Tetrahydrocannabinol Derivative. American Journal of Therapeutics, 2(10), 799–805. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11854790
Other references
Allan, G. M., Ramji, J., Perry, D., Ton, J., Beahm, N. P., Crisp, N., … Lindblad, A. J. (2018). Simplified guideline for prescribing medical cannabinoids in primary care. Canadian Family Physician, 64(2).
National Center for Environmental Health. (n.d.). Synthetic cannabinoids: What are they? What are their effects? | HSB | NCEH. Retrieved March 29, 2019, from https://www.cdc.gov/nceh/hsb/chemicals/sc/default.html
Villan, S. (2008). Use of Δ9-tetrahydrocannabinol in the treatment of spasticity in spinal cord injury patients. Spinal Cord, 46(6), 460–460. https://doi.org/10.1038/sj.sc.3102149
Image credits
- Marijuana ©United States Fish and Wildlife Service, CC0 1.0
- Image by SCIRE Community Team
- Cannabidiol and THC Biosynthesis ©Madkamin, CC BY-SA 4.0
- Weeds ©The Other Dan, CC BY-NC 2.0
- ‘Spice’ — a designer synthetic cannabinoid ©G.W. Pomeroy, CC0 1.0
- Vape Pen ©Aly Dodds, CC BY 3.0 US
- Cannabis Pills ©Mooms, CC BY 3.0 US
- CBD Oil ©Mooms, CC BY 3.0 US
- Cannabis Cream ©Mooms, CC BY 3.0 US
- When in Amsterdam… ©ashton, CC BY 2.0
- CBDistillery-OIL-benefits ©Robert Fischer, CC BY-NC 2.0
- Hmmmm cannabis ©Steven Schwartz, CC BY 2.0
- Bodily effects of cannabis ©Mikael Häggström, CC0 1.0
- Marijuana side effect ©dDara, CC BY 3.0 US
- Marijuana side effect ©dDara, CC BY 3.0 US








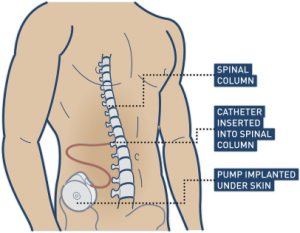

 Botulinum toxin is given with an injection into the affected spastic muscle or bladder.
Botulinum toxin is given with an injection into the affected spastic muscle or bladder.