Authors: Sharon Jang, Vanessa Mok, Dominik Zbogar | Reviewer: Phillip Popovich | Published: 5 May 2020 | Updated: ~
The gut microbiome, also known as gut flora or gut microbiota, refers to the organisms that live in our digestive system. Research suggests that changes to the gut microbiome can affect the development of long-term complications and recovery following spinal cord injury (SCI).
Key Points
- The microbiome is a community of organisms in the gut that contribute to the body’s day-to-day functions
- Factors such as diet, medications, physical activity, sleep, smoking, and stress have been shown to affect the balance of the gut microbiome in the general population.
- After SCI, the gut microbiome experiences unique challenges and changes. The implications of these changes are poorly understood.
- Currently, very few studies exist regarding the gut microbiome in people with SCI.
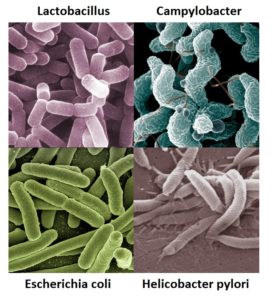
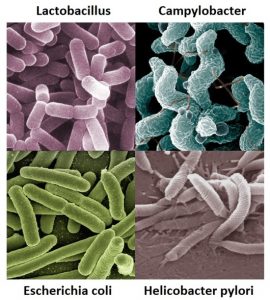
Examples of bacteria that can be found in the digestive system.1-4
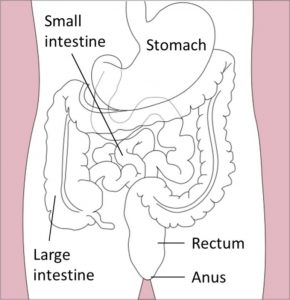
The microbiome refers to the trillions of viruses, fungi, and bacteria living all over the body. Although you may see the terms “microbiota” and “microbiome” sometimes used interchangeably, the microbiome technically refers to the genetic makeup (i.e. the DNA) of these organisms, while the microbiota refers to the organisms themselves. The core microbiota in humans is similar between people, however each of us has their own distinct variation of bacteria, viruses, and fungi that comprises their microbiota. These organisms exist in many areas of the body, including on the skin, in the nose, in the vagina, and in the bladder. However, microbiota have the highest density and variation in the large intestine.
Our past view of microbiota was focused on their potential to cause infection. However, more recent research has shown that microbiota play a substantial role in normal development and daily body functions. The greater the diversity or variation of the microbiota, the healthier and more resilient it is. The opposite has been associated with negative long-term effects on diseases later in life. Although research on the microbiome is still emerging, researchers have discovered that the microbiome is responsible for:
- Preventing the growth of other harmful organisms
- Stimulating the immune system to help fight off infections
- Preventing the development of allergies
- Food digestion and nutrient absorption
- Sugar and fat metabolism
- Brain development
- Drug metabolism
In recent years, the microbiome has gained a lot of interest in research. This is partly due to new technology which allow scientists to observe the DNA of bacteria, resulting in a more specific analysis. Bacteria is primarily prevalent in the intestines, and amount to 10 times more than all the cells in the human body. This adds up to 1-3% of body mass, or 2-6 lbs of your weight. This article will focus on the gut microbiota, specifically the bacterial component of the microbiota, as most of the research on the microbiome thus far has focused on bacteria rather than fungi and viruses.
Development of the gut microbiome
|
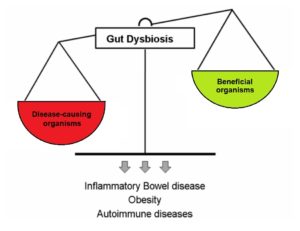
Dysbiosis occurs when disease-causing organisms become more dominant than beneficial organisms.6
The microbiota is comprised of both good and bad bacteria, as well as bacteria that may be good or bad depending on the environment and circumstances. When your body is healthy, the good bacteria are able to keep pathogens in check, thus preventing illness. However, there are certain situations when disease-causing organisms become more prevalent than the beneficial bacteria. Known as dysbiosis, this state in which the microbiota is not in balance may be caused by stress, antibiotic use, and dysfunction of the intestines. Traumatic SCI can cause neurological and psychological complications which require care that may predispose these individuals to dysbiosis. These include:
- Psychological stress after injury and during adaptation
- Having a neurogenic bowel/bladder
- A weakened immune system, which creates a greater need for antibiotics
Dysbiosis can also lead to short-term negative effects such as food intolerances, stomach upset, and an increased risk of developing infections. It is also linked to chronic conditions such as:
- Allergies
- Psychiatric conditions (e.g. depression, anxiety)
- Autoimmune diseases (e.g. rheumatoid arthritis, Crohn’s disease, inflammatory bowel disease)
- Metabolic disorders (e.g. obesity, diabetes)
- Neurologic conditions (e.g. pain, Alzheimer’s, neurogenic bowel dysfunction)
- Non-alcoholic fatty liver disease
Many of these conditions are already common in people living with SCI. Hence, a healthy microbiome may be important in maintaining the regular functions of the microbiome and preventing the consequences of dysbiosis following SCI.
Movement of the gut slows as a consequence of neurogenic bowel, which is a common in SCI, especially with higher levels of injury.7
After SCI, one of the main group of nerves that innervates the gut, the sympathetic nervous system, becomes impaired. This can impact the gut microbiota in 3 ways: through slowing gut movement, through modifying the ability for microbiota to thrive, and through modifying the immune system in the gut. The slower movement of the gut contents, one of the results of impaired bowel function (known as neurogenic bowel dysfunction) after SCI, can impact the microbiota at the far end of the intestines by delaying the delivery of important nutrients. This is of concern because bacteria in the gut thrive by fermenting or breaking down foods such as starches and fibre, and produce metabolites such as butyrate. One study with weak evidence found that people with chronic (at least 12 months post-injury) complete SCI had significant decreases in butyrate-producing bacteria compared to the non-SCI population. As butyrate has anti-inflammatory effects on the nervous system, researchers believe that low butyrate levels can negatively affect long-term recovery following SCI due to increased inflammation.
Millions of bacteria grow on the mucus in the gut, forming a biofilm.8
Secondly, the sympathetic nervous system is responsible for stimulating mucus secretion within the gut. With a lack of input from the sympathetic nervous system, the production of mucus is decreased. This has important implications for the microbiota in the intestines, as mucus acts as a surface that allows for bacteria to bind to it, thus creating a biofilm. A biofilm is a group of bacteria that has formed a structured community on a surface. With a reduced amount of area for bacteria to thrive, the types of bacteria living in the gut may be altered.
Thirdly, the gut has a protective barrier to prevent bad bacteria from entering the body through the intestinal walls. This immune system within the gut is known as the gastrointestinal-associated lymphoid tissue (GALT) and is controlled by the sympathetic nervous system. With a lack of signaling from the sympathetic nervous system, functioning of the GALT may become compromised. In addition, chronic stress or trauma (which can be brought on by SCI) may change the permeability of the wall of the intestine, allowing harmful bacteria into the body. This may be a potential source of inflammation following SCI and may partly explain why people with SCI are more prone to long-term complications. While research with SCI rat models shows this compromise of the protective barrier and movement of bacteria into places it does not usually reside, (like the blood), it is not yet known if the same is true for humans.
Individuals with SCI are found to have different bacteria comprising their microbiota, compared to able-bodied individuals. For example, one study (weak evidence) found that the diversity of gut bacteria and number of overall bacteria of individuals with chronic tetraplegia were less than in able-bodied individuals. Conversely, another study found individuals with SCI to harbour greater bacterial diversity in their gut relative to people without SCI. However, the greater variation of bacteria in people with SCI consist of bacteria less commonly found in able-bodied individuals. The implications of these differences are unknown and have yet to be researched.

Comparing gut bacteria between different populationsDifferences in bacterial composition have been noted in people with other health conditions (such as schizophrenia and diabetes), but whether these differences contributed to the progression of the disease or arose due to the condition is unclear. Also, differences between countries exist and may be attributed to differences in environmental conditions (e.g. diet, lifestyle). However, impacts of these alterations are largely unknown. |
 Previously, it was thought that urine was clean and sterile – that urine should be free from bacteria and white blood cells, which are both signs of infection. In recent years, researchers have discovered that healthy urine is not always sterile. In a conditioned called asymptomatic bacteriuria, non-harmful bacteria are found in the urine, but the individual does not experience any symptoms of a urinary tract infection (UTI) or other illness. Although certain strains of bacteria in urine may be healthy, after SCI, the proportion and composition of bacteria found in urine changes. One (weak evidence) study suggests that individuals with SCI and neurogenic bladders have microbiomes with more unhealthy bacteria, which may be a precursor for UTIs.
Previously, it was thought that urine was clean and sterile – that urine should be free from bacteria and white blood cells, which are both signs of infection. In recent years, researchers have discovered that healthy urine is not always sterile. In a conditioned called asymptomatic bacteriuria, non-harmful bacteria are found in the urine, but the individual does not experience any symptoms of a urinary tract infection (UTI) or other illness. Although certain strains of bacteria in urine may be healthy, after SCI, the proportion and composition of bacteria found in urine changes. One (weak evidence) study suggests that individuals with SCI and neurogenic bladders have microbiomes with more unhealthy bacteria, which may be a precursor for UTIs.
With the knowledge that pre-existing bacteria live in the bladder, some researchers have questioned whether it would be possible to modify the microbiota to prevent UTIs. Bacterial interference is a process whereby non-harmful bacteria are injected into the bladder. Ideally, the benign bacteria prevent the growth of harmful bacteria by creating competition for nutrients and space to colonize. Multiple studies (weak evidence) indicate bacterial interference may decrease the occurrence of UTIs in individuals with SCI, and may delay the recurrence of UTIs. However, there are barriers to using bacterial interference, including:
- The process of injecting bacteria into the bladder, which is a cumbersome process that requires multiple administrations over consecutive days.
- The maintenance of the injected bacteria, which may not successfully colonize the bladder.
Given these limitations to bacterial interference in the bladder, other researchers have attempted to change the microbiome through indwelling catheters. Individuals who use indwelling catheters are at risk for UTIs, as bacteria can grow in the catheter and make its way back up into the bladder. To counter this, some researchers have observed the impact of coating indwelling catheters with non-harmful bacteria in attempts to reduce rates of UTI. Using the concept of bacterial interference again, the purpose of coating an indwelling catheter is to prevent harmful bacteria from growing inside it, thus preserving the bladder microbiota. These studies (weak evidence) found this method decreased the average number of UTIs experienced per year, and that using bacterial interference in catheters is a successful strategy for preventing the growth of harmful bacteria.
Changes to the vaginal microbiome after SCIThe vaginal microbiota is primarily dominated by Lactobacilli bacteria, which act as a first line of defence against harmful bacteria. This is done by Lactobacilli creating an acidic environment, competing for nutrients and growth sites, and stimulating the immune system. As a result, the vagina is protected from the growth of harmful bacteria, including those causing sexually transmitted infections (STIs). In one study (weak evidence), researchers found that women with SCI have less Lactobacilli bacteria and more bacteria associated with UTIs and yeast infections. Overall, there is limited evidence demonstrating microbiome differences in the urinary and reproductive systems of people with SCI. More research is needed to determine the implications of these variations and whether intervention is beneficial or necessary. |
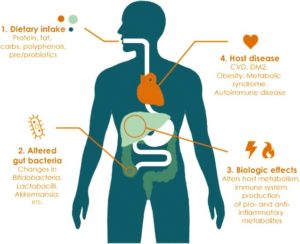
Lifestyle factors can have a large impact on the bacterial composition in the gut. This in turn can cause biological changes that may predispose an individual to long-term diseases.11
The numerous factors known to affect the balance of the gut microbiome, and their relationship to SCI are discussed below.
Diet
Diet has a large impact on the composition of the bacteria in the gut. In particular, processed foods in the Western diet may contribute to decreases in microbiome function and diversity. Conversely, a diet that is comprised of a variety of whole foods and probiotics can promote a diverse community of bacteria. Many studies with weak to moderate evidence have demonstrated an association between the bacterial community in the gut and the development of diseases such as diabetes and obesity. However, there is still much to learn about the functions of the gut microbiome before we can find an optimal approach to diet that will control microbiome-induced chronic diseases.
Alcohol

Drinking alcohol can affect the integrity of the gut microbiome, but these effects may be dependent on the type of alcohol consumed. One study in able-bodied males found that those who consumed gin had decreased numbers of beneficial gut bacteria. On the other hand, those who consumed red wine had decreased numbers of harmful bacteria and increased numbers of beneficial bacteria. The researchers believed that moderate consumption of red wine provided a source of polyphenols which may explain differences seen between the two groups. Polyphenols are compounds found in plants that may have prebiotic-like effects. While the effects of alcohol on the microbiome in SCI are unknown, alcohol should be limited to avoid the known health risks on other body systems.
Medications
Antibiotics are a well-known class of drugs that can disrupt the growth of both harmful and beneficial organisms in the gut. This can effectively decrease the number and diversity of the bacteria. Susceptibility to urinary tract and lung infections and pressure ulcers is increased following SCI. Because antibiotics are frequently used to treat these conditions, people with SCI may experience further disruptions to an already disrupted microbiome. Some may also report fatigue, emotional, or neurological issues with antibiotic use, suggesting that what is happening in the gut can have an effect in other parts of the body.
Physical Activity
 There is large variability in the amount of exercise performed by people with SCI, but most do not engage in any. Various studies on able-bodied individuals report a greater amount of certain healthy gut bacteria in physically active people compared to less active people. Thus, in SCI there may be a missed opportunity for physically inactive people to benefit from these healthy bacteria in their gut, some of which have anti-inflammatory effects and can protect from obesity.
There is large variability in the amount of exercise performed by people with SCI, but most do not engage in any. Various studies on able-bodied individuals report a greater amount of certain healthy gut bacteria in physically active people compared to less active people. Thus, in SCI there may be a missed opportunity for physically inactive people to benefit from these healthy bacteria in their gut, some of which have anti-inflammatory effects and can protect from obesity.
Refer to our chapter on Exercise Guidelines to learn more about physical activity following SCI.
Sleep
Sleep problems occur more commonly in individuals with SCI compared with the general population. This is important because sleep deprivation can cause disruptions in cognition, immune function, and many other body functions. A recent study looked at how the lack of sleep impacted bacteria in the gut among the general population. It found that just two consecutive days of sleep deprivation lead to increased amounts of bacteria that are implicated in conditions such as weight gain and diabetes. These conditions are already prevalent in people living with SCI.
Smoking

Smoking can lead to unwanted effects on the gut microbiome.14
There is evidence that smoking can cause negative changes in the diversity of the gut microbiome. Notably, the bacterial composition changes caused by smoking appear to be similar to the changes brought about by conditions like inflammatory bowel disease and obesity. Not only does smoking upset the balance of the microbiome, but it can lead to many short-term and long-term consequences like pneumonia which is especially harmful in people with SCI who have pre-existing breathing problems.
Stress
Recent evidence suggests a link between stress and negative changes in the gut microbiome. Stress may play a role in gut bacteria disruptions following spinal cord injury as people with SCI experience both massive physical stress from the injury itself and significant psychological stress due to dramatic life changes.
There is limited data on interventions that affect the gut microbiome in the SCI population. Proposed interventions include the use of probiotics, prebiotics, and fecal transplantation.
Probiotics
 Probiotics are live organisms that can be consumed to replenish the microbiome. Probiotics are available as supplements and are often present in cultured/fermented food products like yogurt, kefir, sauerkraut, kimchi, and miso. However, some of these food sources contain high levels of salt, saturated fat, or other ingredients associated with health risks, so ensuring moderation and variety of these types of food is important. Often times, many of these products to not contain large enough concentrations of probiotic bacteria to have a meaningful benefit. Also, production of supplements is not government regulated so there is no way to know if the amount and type of bacteria listed on a supplement label is actually found in that product.
Probiotics are live organisms that can be consumed to replenish the microbiome. Probiotics are available as supplements and are often present in cultured/fermented food products like yogurt, kefir, sauerkraut, kimchi, and miso. However, some of these food sources contain high levels of salt, saturated fat, or other ingredients associated with health risks, so ensuring moderation and variety of these types of food is important. Often times, many of these products to not contain large enough concentrations of probiotic bacteria to have a meaningful benefit. Also, production of supplements is not government regulated so there is no way to know if the amount and type of bacteria listed on a supplement label is actually found in that product.
Individuals with SCI frequently receive antibiotics given their increased risk of bacterial infections and this puts people with SCI at risk for antibiotic-associated diarrhea. One study with moderate evidence showed that probiotic drinks may prevent dysbiosis in people with SCI who are at risk of antibiotic-associated diarrhea. Another study with moderate evidence supported the use of probiotics after SCI as treatment for antibiotic-associated diarrhea by shortening the course of diarrhea by about 2 days. More research is needed to confirm these results and determine the safety and effectiveness of probiotics in people with SCI.
Antibiotic-associated diarrhea vs. C. difficile-associated diarrhea The bacterium Clostridium difficile is responsible for C. difficile-associated diarrhea.16 Antibiotic-associated diarrhea is defined as the passing of three or more loose, watery stools per day due to taking oral antibiotics. This form of diarrhea generally requires little to no treatment and stops after discontinuation of the antibiotics. Antibiotics can cause dysbiosis in the gut, leading to increased vulnerability to Clostridium difficile bacteria (also known as C. difficile or C. diff). This pathogenic bacterium releases a toxin that can cause diarrhea as well as other signs of infection such as fever and inflammation. This form of diarrhea, known as C. difficile-associated diarrhea requires treatment to get rid of the bacteria |
Prebiotics
Prebiotics are dietary substances that are fermented or broken down by bacteria. In this way, prebiotics support the growth of beneficial gut bacteria by acting as a nutrient source. Foods high in fermentable fibres contain prebiotics. Examples of prebiotic-containing sources include whole grain products like barley as well as fruits and vegetables like bananas, onions, and asparagus.
The prebiotic lactulose is widely known as a treatment for constipation and hepatic encephalopathy (decreased brain function due to liver failure). Research suggests that prebiotics may also be a potential treatment for irritable bowel disease, but less is known about the use of prebiotics in other conditions. Although consuming 5-20 g/day of prebiotics was shown to significantly increase gut bacteria, there is insufficient data on the use of prebiotics in SCI to draw any conclusions at this time
Fecal transplantation
In one case study (weak evidence), a male with tetraplegia experiencing recurrent C. difficile infection was successfully treated with antibiotics following fecal transplantation (also known as fecal microbiota transplant or FMT). The researchers reported that transplants given through the oral route may be more feasible to avoid adverse effects related to transplants given through colonoscopies.
In another case study (weak evidence) of a male with tetraplegia, fecal transplantation resulted in resolution of C. difficile infection. Other noted benefits following his fecal transplant included a reduction in antibiotic-resistant organisms, episodes of sepsis (blood poisoning), infections, and antibiotic use.
What are fecal transplants Stool can be freeze-dried and placed into capsules in preparation for fecal transplantation.17 Fecal transplantation involves the replacement of gut bacteria by transferring stool from a healthy donor into the digestive tract of the recipient. Delivery methods include enemas, oral capsules of frozen stool, colonoscopies, rectal tubes, or feeding tubes. Fecal transplants are currently used as a treatment option after multiple failed courses of antibiotics in people with recurrent infections due to C. difficile bacteria. It is believed that introducing stool containing organisms into a recipient’s gut enhances microbiome diversity and increases resistance to pathogenic organisms. However, fecal transplants are not without risk. Side effects that have been reported in studies include constipation, diarrhea, stomach upset, and fever. Other negative effects may result from the endoscopy procedure (e.g. bleeding) or the transmission of pathogenic organisms (ex. infection). Fecal transplantation has been gaining attention as a potential management option for other conditions like inflammatory bowel disease, inflammatory bowel syndrome, hepatic encephalopathy, autism, metabolic syndrome, and obesity. Long-term safety data is not well-defined, but with the surge of ongoing studies, this gap may be filled in the near future. |
Differences in bacterial composition have been identified in people with SCI and people without. As dysbiosis has been linked to various chronic diseases, maintaining a healthy gut microbiome may be a target to prevent or reduce the development of many long-term complications that develop after SCI.
There are currently limited studies on interventions affecting the gut microbiome in people with SCI. Diet has been demonstrated to have a significant role in shaping the microbiome and can be optimized by eating a healthy, plant-based diet. Probiotics may also be consumed to counteract some negative effects of antibiotics. Research in the able-bodied population also suggests that engaging in regular physical activity, achieving adequate sleep, avoiding smoking, and reducing stress may be key in supporting good bacteria and reducing bad gut bacteria.
We have only just begun to scratch the surface of how important the role of the microbiome is in various functions, and its relationship with genetics and the physical environment. Until more research evidence is available, the best course of action is to consult your health provider to find out how lifestyle factors can be modified to enhance the health of your microbiome.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Evidence for “What is the gut microbiome” is based on:
Valdes, A. M., Walter, J., Segal, E., & Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. BMJ (Online), 361, 36–44. https://doi.org/10.1136/bmj.k2179
Rescigno, M. (2014). Intestinal microbiota and its effects on the immune system. Cellular Microbiology, 16(7), 1004–1013. https://doi.org/10.1111/cmi.12301
Darouiche, R. O., & Hull, R. A. (2012). Bacterial interference for prevention of urinary tract infection. Clinical Infectious Diseases, 55(10), 1400–1407. https://doi.org/10.1093/cid/cis639
Rescigno, M. (2014). Intestinal microbiota and its effects on the immune system. Cellular Microbiology, 16(7), 1004–1013. https://doi.org/10.1111/cmi.12301
Ma, B.; Forney, L.; Ravel, J. (2013). The vaginal microbiome: rethinking health and diseases, 371–389.
Bull, M. J., & Plummer, N. T. (2014). Part 1: The Human Gut Microbiome in Health and Disease. Integrative medicine (Encinitas, Calif.), 13(6), 17–22
Bäckhed, F., Ding, H., Wang, T., Hooper, L. V, Koh, G. Y., Nagy, A., … Gordon, J. I. (2004). The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America, 101(44), 15718–15723. https://doi.org/10.1073/pnas.0407076101
Kigerl, K. A., Hall, J. C. E., Wang, L., Mo, X., Yu, Z., & Popovich, P. G. (2016). Gut dysbiosis impairs recovery after spinal cord injury. The Journal of Experimental Medicine, 213(12), 2603–2620. https://doi.org/10.1084/jem.20151345
Collins, S. M., Surette, M., & Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology, 10(11), 735–742. https://doi.org/10.1038/nrmicro2876
Jandhyala, S., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., & Reddy, D. (2015). Role of the normal gut microbiota. World Journal of Gastroenterology, 21(29), 8836–8847. https://doi: 10.3748/wjg.v21.i29.8787
Linsenmeyer, T. (2018). Catheter-associated urinary tract infections in persons with neurogenic bladders. Journal of Spinal Cord Medicine, 41(2), 132-141. https://doi.org/10.1080/10790268.2017.1415419
Tanaka, M., & Nakayama, J. (2017). Development of the gut microbiota in infancy and its impact on health in later life. Allergology International, 66(4), 515–522. https://doi.org/10.1016/j.alit.2017.07.010
Voreades, N., Kozil, A., & Weir, T. L. (2014). Diet and the development of the human intestinal microbiome. Frontiers in Microbiology, 5, 494. https://doi.org/10.3389/fmicb.2014.00494
Evidence for “What happens when the gut microbiome is out of balance?” is based on:
Kigerl, K.A., & Popovich, P.G. (2019). Gut Dysbiosis and Recovery of Function After Spinal Cord Injury. Oxford Research Encyclopedia of Neuroscience. https://doi.org/10.1093/acrefore/9780190264086.013.242
Cao, S., Feehley, T. J., & Nagler, C. R. (2014). The role of commensal bacteria in the regulation of sensitization to food allergens. FEBS Letters, 588(22), 4258–4266. https://doi.org/10.1016/j.febslet.2014.04.026
Foster, J. A., & McVey Neufeld, K.-A. (2013). Gut–brain axis: how the microbiome influences anxiety and depression. Trends in Neurosciences, 36(5), 305–312. https://doi.org/10.1016/j.tins.2013.01.005
Maeda, Y., & Takeda, K. (2017). Role of Gut Microbiota in Rheumatoid Arthritis. Journal of Clinical Medicine, 6(6). https://doi.org/10.3390/jcm6060060
Hold, G. L., Smith, M., Grange, C., Watt, E. R., El-Omar, E. M., & Mukhopadhya, I. (2014). Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World Journal of Gastroenterology, 20(5), 1192–1210. https://doi.org/10.3748/wjg.v20.i5.1192
Castaner, O., Goday, A., Park, Y.-M., Lee, S.-H., Magkos, F., Shiow, S.-A. T. E., & Schröder, H. (2018). The Gut Microbiome Profile in Obesity: A Systematic Review. International Journal of Endocrinology, 2018, 1–9. https://doi.org/10.1155/2018/4095789
Aw, W., & Fukuda, S. (2018). Understanding the role of the gut ecosystem in diabetes mellitus. Journal of Diabetes Investigation, 9(1), 5–12. https://doi.org/10.1111/jdi.12673
Tilg, H., Kaser, A. (2011). Gut microbiome, obesity, and metabolic dysfunction. Journal of Clinical Investigation, 121(6), 2126-32. https://doi.org/10.1172/JCI58109
Murri, M., Leiva, I., Gomez-Zumaquero, J. M., Tinahones, F. J., Cardona, F., Soriguer, F., & Queipo-Ortuño, M. I. (2013). Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Medicine, 11(1), 46. https://doi.org/10.1186/1741-7015-11-46
Rousseaux, C., Thuru, X., Gelot, A., Barnich, N., Neut, C., Dubuquoy, L., … Desreumaux, P. (2007). Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature Medicine, 13(1), 35–37. https://doi.org/10.1038/nm1521
Jiang, C., Li, G., Huang, P., Liu, Z., & Zhao, B. (2017). The Gut Microbiota and Alzheimer’s Disease. Journal of Alzheimer’s Disease, 58(1), 1–15. https://doi.org/10.3233/JAD-161141
Zhang, C., Zhang, W., Zhang, J., Jing, Y., Yang, M., Du, L., … Li, J. J. J. (2018). Gut microbiota dysbiosis in male patients with chronic traumatic complete spinal cord injury. Journal of Translational Medicine, 16(1), 353. https://doi.org/10.1186/s12967-018-1735-9
Waldman, A. J., & Balskus, E. P. (2018). The Human Microbiota, Infectious Disease, and Global Health: Challenges and Opportunities. ACS Infectious Diseases, 4(1), 14–26. https://doi.org/10.1021/acsinfecdis.7b00232
Myers, J., Lee, M., & Kiratli, J. (2007). Cardiovascular Disease in Spinal Cord Injury. American Journal of Physical Medicine & Rehabilitation, 86(2), 142–152. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17251696
Sauerbeck, A.D., Laws, J.L., Bandaru, V.V., Popovich, P.G., Haughey, N.J., & McTigue, D.M. (2015). Spinal cord injury causes chronic liver pathology in rats. Journal of Neurotrauma, 32(3), 159–169. https://doi.org/10.1089/neu.2014.3497
Boekamp, J. R., Overholser, J. C., & Schubert, D. S. P. (1996). Depression following a Spinal Cord Injury. The International Journal of Psychiatry in Medicine, 26(3), 329–349. https://doi.org/10.2190/CMU6-24AH-E4JG-8KBN
Elliott, T. R., & Frank, R. G. (1996). Depression following spinal cord injury. Archives of Physical Medicine and Rehabilitation, 77(8), 816–823. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8702378
Mehta, S., Robert, M. A., Loh, E., Short, C., Frcpc, M. D., Wolfe, D. L., … Msc, H. (2013). Pain Following Spinal Cord Injury. Retrieved from www.scireproject.com
Evidence for “What changes occur in the gut microbiome after SCI?” is based on:
Kigerl, K.A., Zane, K., Adams, K., Sullivan, M.B., & Popovich, P.G. (2020). The spinal cord-gut-immune axis as a master regulator of health and neurological function after spinal cord injury. Experimental Neurology, 323, 113085. https://doi.org/10.1016/j.expneurol.2019.113085
Gungor, B., Adiguzel, E., Gursel, I., Yilmaz, B., & Gursel, M. (2016). Intestinal Microbiota in Patients with Spinal Cord Injury. PLOS ONE, 11(1), e0145878. https://doi.org/10.1371/journal.pone.0145878
Choong, S., & Whitfield, H. (2000). Biofilms and their role in infections in urology. BJU International, 86, 935–941
Noller, C.M., Groah, S.L., Nash, M.S. (2017). Inflammatory Stress Effects on Health and Function After Spinal Cord Injury. Topics in Spinal Cord Injury Rehabilitation, 23(3), 207–217. https://doi.org/10.1310/sci2303-207
Liu, J., An, H., Jiang, D., Huang, W., Zou, H., Meng, C., Li, H. (2004). Study of bacterial translocation from gut after paraplegia caused by spinal cord injury in rats. Spine, 29(2):164–169. https://doi.org/10.1097/01.BRS.0000107234.74249.CD
Kigerl, K. A., Hall, J. C. E., Wang, L., Mo, X., Yu, Z., & Popovich, P. G. (2016). Gut dysbiosis impairs recovery after spinal cord injury. The Journal of Experimental Medicine, 213(12), 2603–2620. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2781092
Zhang, C., Zhang, W., Zhang, J., Jing, Y., Yang, M., Du, L., … Li, J. (2018). Gut microbiota dysbiosis in male patients with chronic traumatic complete spinal cord injury. Journal of Translational Medicine, 16(1), 353. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/30545398
Jeffrey, E. (2018). Investigation of Spinal Cord Injury-Induced Gastrointestinal Dysfunction and Related Microbiota, Fungal, and Intestinal Alterations in a Rat Model and Humans with Spinal Cord Injury. University of Miami. Retrieved from https://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1738&context=oa_theses
Evidence for “Comparing gut bacteria between different populations” is based on:
Nguyen, T.T., Kosciolek, T., Maldonado, Y., Daly, R.E., Martin, A.S., McDonald, D., … Jeste, D.V. (2019) Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophrenia Research, 204, 23–29. https://doi.org/10.1016/j.schres.2018.09.014
Sedighi, M., Razavi. S., Navab-Moghadam, F., Khamseh, M.E., Alaei-Shahmiri, F., Mehrtash, A., Amirmozafari, N. (2017). Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microbial Pathogenesis, 111, 362–369. https://doi.org/10.1016/j.micpath.2017.08.038
Schnorr, S. L., Candela, M., Rampelli, S., Centanni, M., Consolandi, C., Basaglia, G., … Crittenden, A. N. (2014). Gut microbiome of the Hadza hunter-gatherers. Nature Communications, 5, 3654. https://doi.org/10.1038/ncomms4654
Martínez, I., Stegen, J. C., Maldonado-Gómez, M. X., Eren, A. M., Siba, P. M., Greenhill, A. R., & Walter, J. (2015). The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Reports, 11(4), 527–538. https://doi.org/10.1016/j.celrep.2015.03.049
De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J.B., Massart, S., Collini, S., Pieraccini, G., Lionetti, P.. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America, 107, 33, 14691–14696. https://doi.org/10.1073/pnas.1005963107
Evidence for “What changes occur to the bladder microbiome after SCI?” is based on:
Groah, S. L., Pérez-Losada, M., Caldovic, L., Ljungberg, I. H., Sprague, B. M., Castro-Nallar, E., … Pohl, H. G. (2016). Redefining Healthy Urine: A Cross-Sectional Exploratory Metagenomic Study of People With and Without Bladder Dysfunction. Journal of Urology, 196(2), 579–587. https://doi.org/10.1016/j.juro.2016.01.088
Fouts, D. E., Pieper, R., Szpakowski, S., Pohl, H., Knoblach, S., Suh, M.-J., … Groah, S. L. (2012). Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. Journal of Translational Medicine, 10, 174. https://doi.org/10.1186/1479-5876-10-174
Darouiche, R. O., & Hull, R. A. (2012). Bacterial interference for prevention of urinary tract infection. Clinical Infectious Diseases, 55(10), 1400–1407. https://doi.org/10.1093/cid/cis639
Bossa, L., Kline, K., McDougald, D., Lee, B. B., & Rice, S. A. (2017). Urinary catheter-associated microbiota change in accordance with treatment and infection status. PloS One, 12(6), e0177633. https://doi.org/10.1371/journal.pone.0177633
Darouiche, R. O., Green, B. G., Donovan, W. H., Chen, D., Schwartz, M., Merritt, J., … Hull, R. A. (2011). Infectious Diseases Multicenter Randomized Controlled Trial of Bacterial Interference for Prevention of Urinary Tract Infection in Patients With Neurogenic Bladder. Infectious Diseases, 78(2), 341–610. https://doi.org/1016/j.urology.2011.03.062
Hull, R., Rudy, D., Donovan, W., Svanborg, C., Wieser, I., Stewart, C., & Darouiche, R. (2000). Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. Journal of Urology, 163(3), 872–877.
Prasad, A., Riosa, S., Darouiche, R. O., & Trautner, B. W. (2009). A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord, 47, 565–569. https://doi.org/10.1038/sc.2008.166
Sundén, F., Håkansson, L., Ljunggren, E., & Wullt, B. (2010). Escherichia coli 83972 Bacteriuria Protects Against Recurrent Lower Urinary Tract Infections in Patients With Incomplete Bladder Emptying. Journal of Urology, 184(1), 179–185. https://doi.org/10.1016/j.juro.2010.03.024
Trautner, B.W., Hull, R.A., & Darouiche, R.O. (2003). Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology, 61(5),1059–1062. https://doi.org/0.1016/s0090-4295(02)02555-4
Ceccarani, C., Foschi, C., Parolin, C., D’Antuono,A., Gaspari, V., Consolandi, C., … Marangoni, A. (2019). Diversity of vaginal microbiome and metabolome during genital infections. Scientific Reports, 9, 14095. https://doi.org/10.1038/s41598-019-50410-x
Evidence for “Changes to the vaginal microbiome after SCI” is based on:
Pires, C.V., Linhares, I.M., Serzedello, F., Fukazawa, E.I., Baracat, E.C., & Witkin, S.S. (2016). Alterations in the Genital Microbiota in Women With Spinal Cord Injury. Obstetrics & Gynecology, 127(2), 273-278. https://doi.org/10.1097/AOG.0000000000001257
Evidence for “What affects the gut microbiome?” is based on:
Coyle, D. (2017). 8 Surprising Things That Harm Your Gut Bacteria. Retrieved February 12, 2019, from https://www.healthline.com/nutrition/8-things-that-harm-gut-bacteria
Makki, K., Deehan, E. C., Walter, J., & Bäckhed, F. (2018, June 13). The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host and Microbe. Cell Press.
Claesson, M. J., Jeffery, I. B., Conde, S., Power, S. E., O’Connor, E. M., Cusack, S., … O’Toole, P. W. (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature, 488(7410), 178–184. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22797518
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., & Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature, 489(7415), 220–230. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22972295
Singer-Englar, T., Barlow, G., & Mathur, R. (2019, January 2). Obesity, diabetes, and the gut microbiome: an updated review. Expert Review of Gastroenterology and Hepatology. Taylor and Francis Ltd.
Górowska-Kowolik, K., & Chobot, A. (2019). The role of gut micorbiome in obesity and diabetes. World Journal of Pediatrics, 15(4), 332–340.
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., … Turnbaugh, P. J. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. Retrieved from http://www.nature.com/articles/nature12820
Queipo-Ortuño, M. I., Boto-Ordóñez, M., Murri, M., Gomez-Zumaquero, J. M., Clemente-Postigo, M., Estruch, R., … Tinahones, F. J. (2012). Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. The American Journal of Clinical Nutrition, 95(6), 1323–1334. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22552027
Evans, C. T., LaVela, S. L., Weaver, F. M., Priebe, M., Sandford, P., Niemiec, P., … Parada, J. P. (2008). Epidemiology of Hospital-Acquired Infections in Veterans With Spinal Cord Injury and Disorder. Infection Control & Hospital Epidemiology, 29(03), 234–242. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18248306
Wolfe, D., McIntyre, A., Ravenek, K., Martin-Ginis, K., Cheung, A. L., Eng, J. J., … Hsieh, J. T. (2014). Physical Activity Participation Levels in SCI. Retrieved February 11, 2019, from https://scireproject.com/evidence/rehabilitation-evidence/physical-activity/increasing-physical-activity-participation-in-sci/physical-sci/
Zbogar, D., Eng, J. J., Miller, W. C., Krassioukov, A. V, & Verrier, M. C. (2016). Physical activity outside of structured therapy during inpatient spinal cord injury rehabilitation. Journal of Neuroengineering and Rehabilitation, 13(1), 99. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27846851
Monda, V., Villano, I., Messina, A., Valenzano, A., Esposito, T., Moscatelli, F., … Messina, G. (2017). Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Medicine and Cellular Longevity, 2017, 1–8. Retrieved from https://www.hindawi.com/journals/omcl/2017/3831972/
Clarke, S. F., Murphy, E. F., O’Sullivan, O., Lucey, A. J., Humphreys, M., Hogan, A., … Cotter, P. D. (2014). Exercise and associated dietary extremes impact on gut microbial diversity. Gut, 63(12), 1913–1920. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25021423
Bressa, C., Bailén-Andrino, M., Pérez-Santiago, J., González-Soltero, R., Pérez, M., Montalvo-Lominchar, M. G., … Larrosa, M. (2017). Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLOS ONE, 12(2), e0171352. Retrieved from https://dx.plos.org/10.1371/journal.pone.0171352
Estaki, M., Pither, J., Baumeister, P., Little, J. P., Gill, S. K., Ghosh, S., … Gibson, D. L. (2016). Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome, 4(1), 42. Retrieved from http://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-016-0189-7
Biering-Sørensen, F., Jennum, P., & Laub, M. (2009). Sleep disordered breathing following spinal cord injury. Respiratory Physiology and Neurobiology, 169(2), 165–170.
Benedict, C., Vogel, H., Jonas, W., Woting, A., Blaut, M., Schürmann, A., & Cedernaes, J. (2016). Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Molecular Metabolism, 5(12), 1175–1186. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27900260
Savin, Z., Kivity, S., Yonath, H., & Yehuda, S. (2018). Smoking and the intestinal microbiome. Archives of Microbiology, 200(5), 677–684. Retrieved from http://link.springer.com/10.1007/s00203-018-1506-2
Stolzmann, K. L., Gagnon, D. R., Brown, R., Tun, C. G., & Garshick, E. (2010). Risk factors for chest illness in chronic spinal cord injury: a prospective study. American Journal of Physical Medicine & Rehabilitation, 89(7), 576–583. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20463565
Clark, A., & Mach, N. (2016). Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. Journal of the International Society of Sports Nutrition, 13(1), 43. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27924137
Tillisch, K., Mayer, E. A., Gupta, A., Gill, Z., Brazeilles, R., Le Nevé, B., … Labus, J. S. (2017). Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosomatic Medicine, 79(8), 905–913. Retrieved from http://insights.ovid.com/crossref?an=00006842-201710000-00010
Knowles, S. R., Nelson, E. A., & Palombo, E. A. (2008). Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: A possible mechanism underlying susceptibility to illness. Biological Psychology, 77(2), 132–137. Retrieved from https://www.sciencedirect.com/science/article/pii/S0301051107001597
Kigerl, K. A., Hall, J. C. E., Wang, L., Mo, X., Yu, Z., & Popovich, P. G. (2016). Gut dysbiosis impairs recovery after spinal cord injury. Journal of Experimental Medicine, 213(12), 2603–2620.
Boekamp, J. R., Overholser, J. C., & Schubert, D. S. P. (1996). Depression following a Spinal Cord Injury. The International Journal of Psychiatry in Medicine, 26(3), 329–349. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8976473
Elliott, T. R., & Frank, R. G. (1996). Depression following spinal cord injury. Archives of Physical Medicine and Rehabilitation, 77(8), 816–823. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8702378
Evidence for “What treatments have a positive effect on the gut microbiome in people with SCI?” is based on:
Wong, S., Jamous, A., O’Driscoll, J., Sekhar, R., Weldon, M., Yau, C. Y., … Forbes, A. (2014). A Lactobacillus casei Shirota probiotic drink reduces antibiotic-associated diarrhoea in patients with spinal cord injuries: A randomised controlled trial. British Journal of Nutrition.
Curtin,P., Casella,G.D., & Turk, M.A. (2017). P7 Can probiotics shorten the duration of antibiotic associated diarrhea in spinal cord injury patients with neurogenic bowel? The Journal of Spinal Cord Medicine, 40(5), 605–625. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28758546
Hod, K., & Ringel, Y. (2017). Treatment of Functional Bowel Disorders With Prebiotics and Probiotics. In The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics, and Dysbiosis (pp. 355–364). Elsevier Inc.
Roberfroid, M. B. (2005). Introducing inulin-type fructans. British Journal of Nutrition, 93(S1), S13–S25.
Tuohy, K. M., Probert, H. M., Smejkal, C. W., & Gibson, G. R. (2003, August 1). Using probiotics and prebiotics to improve gut health. Drug Discovery Today.
Gibson, G. R., Probert, H. M., Loo, J. Van, Rastall, R. A., & Roberfroid, M. B. (2004). Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutrition Research Reviews, 17(2), 259–275.
Brechmann, T., Swol, J., Knop-Hammad, V., Willert, J., Aach, M., Cruciger, O., … Hamsen, U. (2015). Complicated fecal microbiota transplantation in a tetraplegic patient with severe Clostridium difficile infection. World Journal of Gastroenterology, 21(12), 3736–3740.
Crum-Cianflone, N. F., Sullivan, E., & Ballon-Landa, G. (2015). Fecal Microbiota Transplantation and Successful Resolution of Multidrug-Resistant-Organism Colonization. Journal of Clinical Microbiology, 53(6), 1986–1989. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25878340
Khanna, S., Vazquez-Baeza, Y., González, A., Weiss, S., Schmidt, B., Muñiz-Pedrogo, D. A., … Kashyap, P. C. (2017). Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome, 5(1), 55. Retrieved from http://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-017-0269-3
Wang, J. W., Kuo, C. H., Kuo, F. C., Wang, Y. K., Hsu, W. H., Yu, F. J., … Wu, D. C. (2019, March 1). Fecal microbiota transplantation: Review and update. Journal of the Formosan Medical Association. Elsevier B.V.
Rossen, N. G., Fuentes, S., Van Der Spek, M. J., Tijssen, J. G., Hartman, J. H. A., Duflou, A., … Ponsioen, C. Y. (2015). Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology, 149(1), 110-118.e4.
Paramsothy, S., Paramsothy, R., Rubin, D. T., Kamm, M. A., Kaakoush, N. O., Mitchell, H. M., & Castaño-Rodríguez, N. (2017). Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. Journal of Crohn’s and Colitis, 11(10), 1180–1199.
Moayyedi, P., Surette, M. G., Kim, P. T., Libertucci, J., Wolfe, M., Onischi, C., … Lee, C. H. (2015). Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology, 149(1), 102-109.e6.
Johnsen, P. H., Hilpüsch, F., Cavanagh, J. P., Leikanger, I. S., Kolstad, C., Valle, P. C., & Goll, R. (2018). Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. The Lancet Gastroenterology and Hepatology, 3(1), 17–24.
Bajaj, J. S., Kassam, Z., Fagan, A., Gavis, E. A., Liu, E., Cox, I. J., … Gillevet, P. M. (2017). Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology, 66(6), 1727–1738.
Millan, B., Laffin, M., & Madsen, K. (2017, September 1). Fecal Microbiota Transplantation: Beyond Clostridium difficile. Current Infectious Disease Reports. Current Medicine Group LLC 1.
Kootte, R. S., Levin, E., Salojärvi, J., Smits, L. P., Hartstra, A. V., Udayappan, S. D., … Nieuwdorp, M. (2017). Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metabolism, 26(4), 611-619.e6.
Krajicek, E., Fischer, M., Allegretti, J. R., & Kelly, C. R. (2019, January 1). Nuts and Bolts of Fecal Microbiota Transplantation. Clinical Gastroenterology and Hepatology. W.B. Saunders.
Wang, Y., Wiesnoski, D. H., Helmink, B. A., Gopalakrishnan, V., Choi, K., DuPont, H. L., … Jenq, R. R. (2018). Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nature Medicine, 24(12), 1804–1808.
Image credits
- Modified from: Lactobacillus casei ©AJC1, CC BY-SA 2.0
- Modified from: Campylobacter bacteria ©Microbe World, CC BY-NC-SA 2.0
- Modified from: Koli Bacteria ©geralt geralt / 18959 images, CC0 1.0
- Modified from: jpg ©Lamiot, CC0 1.0
- Baby ©Nick Abrams, CC BY 3.0 US
- Modified from: Mazmanian SK, Lee YK. (2014). Interplay between intestinal microbiota and host immune system. Journal of Bacteriology and Virology, 44(1),1-9. CC BY-NC 3.0.
- Intestine segmentation ©Servier Medical Art, CC BY 3.0
- Modified from: Colon ©Servier Medical Art, CC BY 3.0
- The Earth seen from Apollo 17 ©NASA, Public Domain
- Modified from: Bacteria ©Maxim Kulikov, CC BY 3.0 US; urethra ©Prettycons, CC BY 3.0 US; and Zoom Out ©fahmionline, CC BY 3.0 US
- Modified from: Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K. (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 15, 73. CC BY 4.0.
- Red Wine bottle pouring ©Push Doctor, CC BY-NC 2.0
- Army Trials at Fort Bliss 160306-A-QR477-037 ©Adasia Ortiz, CC0 1.0
- Smoke ©Joffrey, CC BY-NC-ND 2.0
- yoghurt pack ©Oleksandr Panasovskyi, CC BY 3.0 US
- Clostridium difficile ©CDC, CC0 1.0
- Freeze-dried poop pills ©Patrik Nygren, CC BY-SA 2.0

 While recent studies have shown that transmission of bacteria from the mother to the fetus is possible and occurs even before birth, it appears that much of the gut microbiome is established during delivery as the baby comes into contact with the microbes present in the mother’s birth canal and skin. Hence, a baby that is born through a C-section will have a different microbial composition. The gut microbiome continues to change due to organisms in the breastmilk; formula-fed infants will present with a different gut microbiome. The diversity of the microbiome increases until it resembles that of an adult around 3 years of age when a solid food diet is established.
While recent studies have shown that transmission of bacteria from the mother to the fetus is possible and occurs even before birth, it appears that much of the gut microbiome is established during delivery as the baby comes into contact with the microbes present in the mother’s birth canal and skin. Hence, a baby that is born through a C-section will have a different microbial composition. The gut microbiome continues to change due to organisms in the breastmilk; formula-fed infants will present with a different gut microbiome. The diversity of the microbiome increases until it resembles that of an adult around 3 years of age when a solid food diet is established.