Authors: Kelsey Zhao, Dominik Zbogar | Published: 14 May 2024
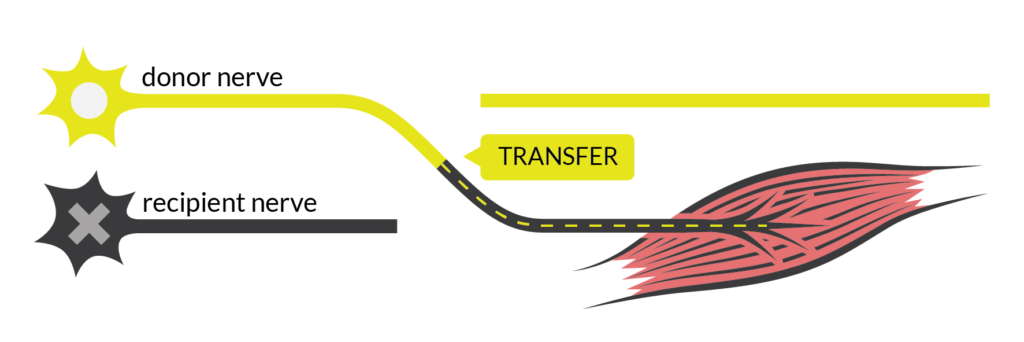
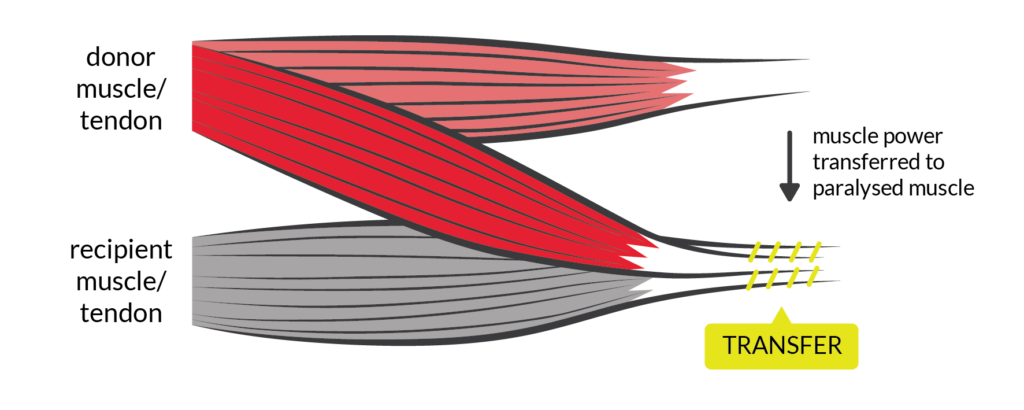
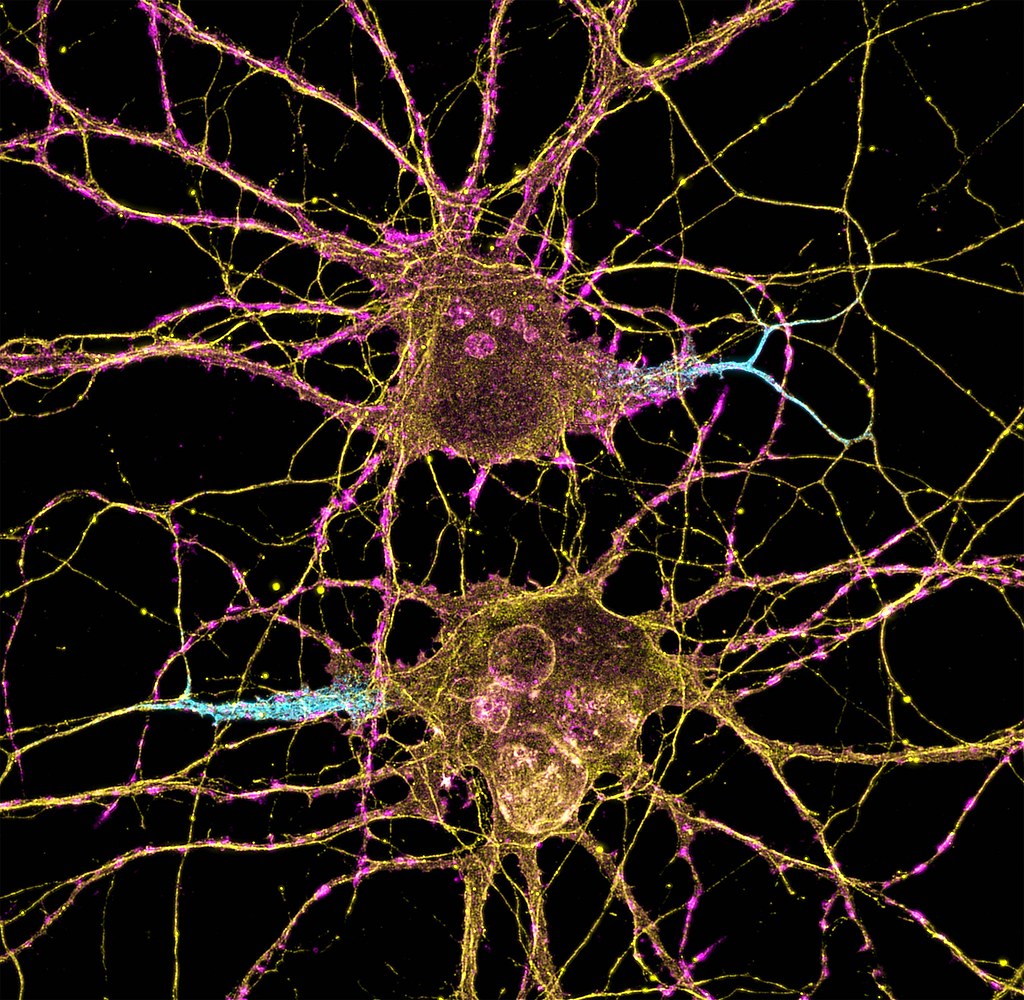
Nerve transfer surgery can restore movement to the paralyzed arm or hand of someone with a high-level spinal cord injury (SCI), by connecting a healthy nerve to the nerve of the paralyzed muscle.
There is much about nerve transfers that we don’t know but we can construct a nuanced view from the diverse experiences of people who have done the procedure. Ainsley, Dan, and Caleb graciously recount their experiences with nerve transfer surgeries, the obstacles they encountered, and the insights they have gleaned, for our readers.
Refer to our article on Nerve Transfer Surgery for more information!
Introducing…
 Ainsley is 17 and plans on doing a Bachelor of Arts at the University of British Columbia after graduating high school this year!
Ainsley is 17 and plans on doing a Bachelor of Arts at the University of British Columbia after graduating high school this year!
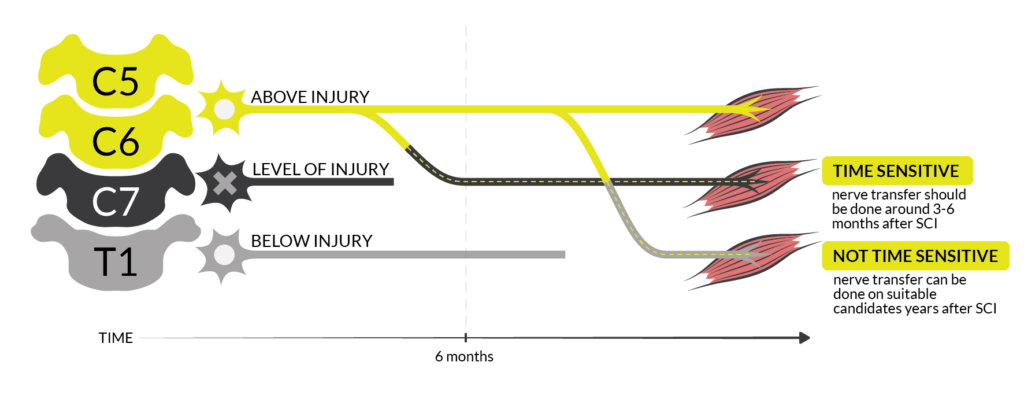
SCI level: C5-C6 complete
 Dan is 37 and a full-time student at Douglas College in Recreation Therapy! He enjoys cooking and has a dog.
Dan is 37 and a full-time student at Douglas College in Recreation Therapy! He enjoys cooking and has a dog.
SCI level: C5-C6 complete
 Caleb is 35 and likes to spend his time outdoors and doing sports like scuba diving, whitewater kayaking and sitskiing!
Caleb is 35 and likes to spend his time outdoors and doing sports like scuba diving, whitewater kayaking and sitskiing!
SCI level: C5 complete
Nerve transfer options
Choosing to have surgery can be a tough decision. There are pros and cons to every procedure and a million factors to consider.
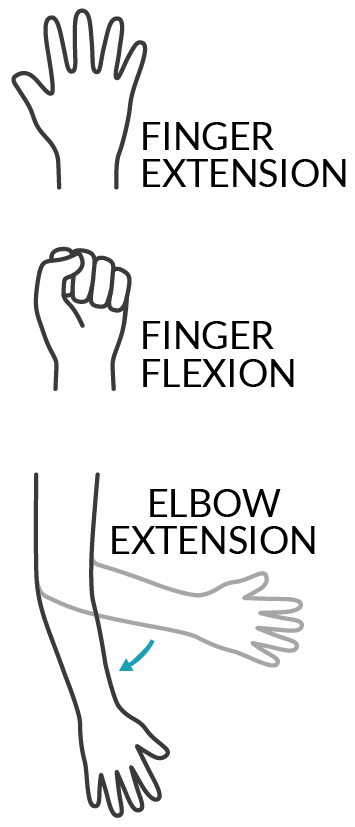
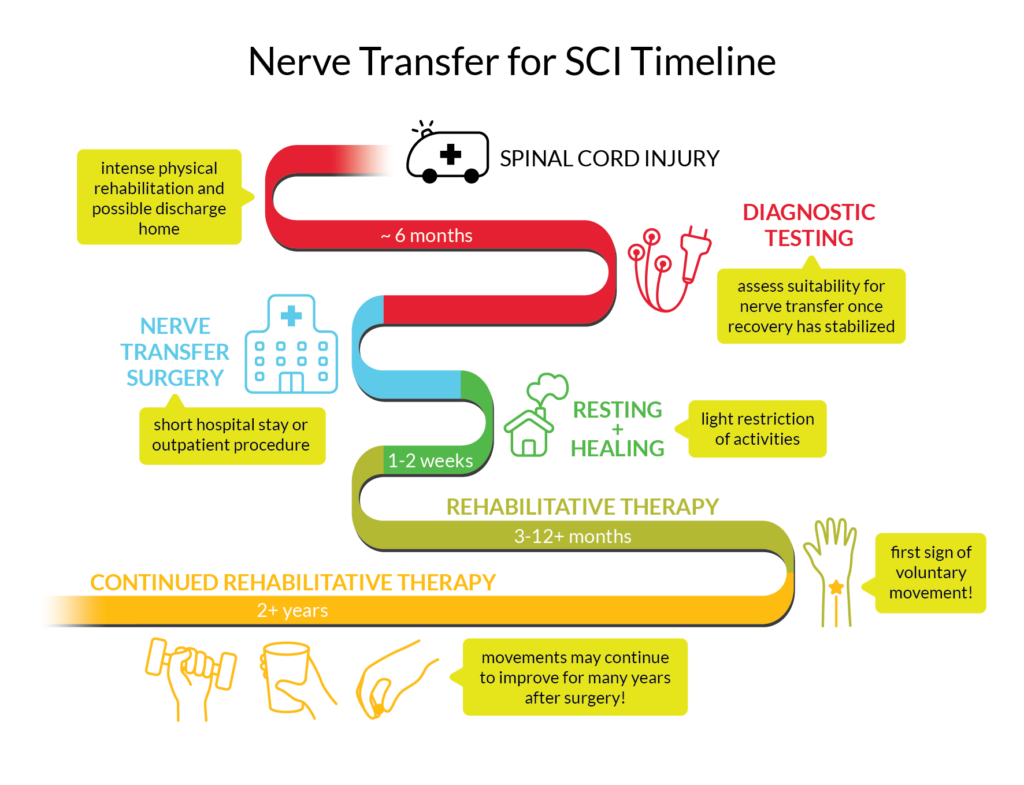
Ainsley opted to have three nerve transfers on each arm: supinator nerve to posterior interosseous nerve (PIN) for hand opening, brachialis nerve to anterior interosseous nerve (AIN)/flexor digitorum superficialis (FDS) for hand closing, and teres minor nerve (with some deltoid) to triceps for elbow extension. Ainsley’s surgical team was able to do her surgeries 6 months after her SCI, during a holiday break from school. Keeping in mind that nerve transfers are not always successful, the nerves were carefully selected to make sure tendon transfers could be done as backups. This precaution paid off when the right-side hand closing nerve transfer didn’t work out.
Dan had two nerve transfers on each arm for finger extension and finger flexion. Unlike Caleb and Ainsley who had nerve transfers done only a few months after injury, Dan had been living with SCI for 5 years when he did the surgeries. Nerve transfer is not always possible for a chronic injury because the muscle might be too deteriorated to recover. However, it can still be an option if electrodiagnostic tests show that there is still activity in the muscle and nerve. Dan said, “they did a test to see whether my nerves were still viable, and they were.”
Caleb had three nerve transfers on both arms 5 months after his cervical SCI: supinator nerve to PIN for finger extension, brachialis nerve to AIN for finger flexion, and deltoid to triceps for elbow extension. In the first few months after his SCI, but before the nerve transfer surgeries, he had recovered good wrist function, but his fingers and triceps were not improving. At that point, he was told what the probability of getting hand function back was and decided that doing the nerve transfers was the best option. Caleb said, “Even if it works out slightly, it will still be better than not doing it”.
Recovery: the good and the bad
Although the evidence so far shows that nerve transfer surgery rarely causes any lasting harms, the general risks that accompany any surgical procedure do exist, and the recovery period can be challenging. Negative experiences do exist alongside the overall success of the procedure.
Ainsley had her nerve transfer surgeries while recovering from SCI at a rehabilitation centre. She stayed at the centre for a few days after the surgery but went home for the holiday season, then returned to continue rehabilitation. After the surgery, there was no cast, splint, or movement restrictions, but the incisions were quite large and painful for the first few days. Over time, the pain became more manageable with pain medication and the stitches dissolved, but it took two or three months before the incision scars stopped bothering her completely. For the first couple of weeks, Ainsley needed a lot of assistance with everyday tasks and had to be careful with big movements like getting dressed. ![]()
Because Ainsley used a power wheelchair, she was able to move around after the surgery like before, but she imagines it would be challenging for someone in a manual chair. Pain and loss of strength after surgery could make pushing a manual wheelchair difficult.
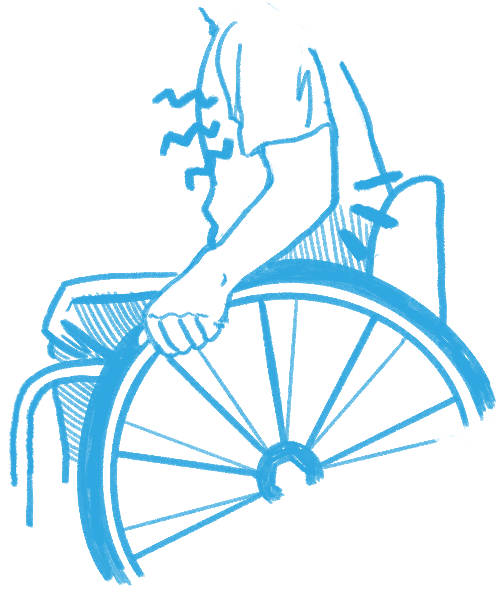 This was very true for Dan. After his nerve transfer surgery, he lost some muscle strength in his left hand and arm. He was still strong enough to push his chair but not to stop. As a result, he went from strictly using a manual chair to using a power wheelchair for about 10 months. The rest of Dan’s recovery did not go so smoothly either. He explains, “in my left arm, when I moved my arm in a certain way, I would get a twang. It felt like I hit my funny bone but times 100. It was really bad and that lasted about two weeks. I also had some numbness in my left thumb all the way down to my palm. I still have numbness but it’s mostly the tip of my thumb so it’s better.” On top of everything, Dan was living at home and not at a rehabilitation centre when he had his surgeries. He came to realize post-surgery that he did not have all the necessary supports in place to accommodate the temporary losses in function. Reflecting on these struggles, he suspects that since people with chronic SCI don’t get nerve transfers often, there is less awareness of how much the surgery can affect their functional abilities.
This was very true for Dan. After his nerve transfer surgery, he lost some muscle strength in his left hand and arm. He was still strong enough to push his chair but not to stop. As a result, he went from strictly using a manual chair to using a power wheelchair for about 10 months. The rest of Dan’s recovery did not go so smoothly either. He explains, “in my left arm, when I moved my arm in a certain way, I would get a twang. It felt like I hit my funny bone but times 100. It was really bad and that lasted about two weeks. I also had some numbness in my left thumb all the way down to my palm. I still have numbness but it’s mostly the tip of my thumb so it’s better.” On top of everything, Dan was living at home and not at a rehabilitation centre when he had his surgeries. He came to realize post-surgery that he did not have all the necessary supports in place to accommodate the temporary losses in function. Reflecting on these struggles, he suspects that since people with chronic SCI don’t get nerve transfers often, there is less awareness of how much the surgery can affect their functional abilities.
Like Ainsley, Caleb was living and recovering at a rehabilitation facility up until when his nerve transfer surgeries were done, and was able to extend his stay a bit to include the first few days of his surgical recovery. He had pain for one day after the nerve transfers were done, followed by the normal aches of surgery. Caleb was still pretty weak from his SCI accident, but he did not feel any difference in strength from before to after the surgery. All in all, nothing unexpected.
What stuck out the most to Caleb about recovery was the amount of time he spent imagining movements (visualization exercises) while no movement was actually happening! Coming from a big sports background, he understood what it meant to visualize actions and the benefits of the exercise. Even so, before the first signs of movement showed up, Caleb had moments where he thought, “Oh man, this is just not doing anything. Will it ever happen?”.
Good to have a back up plan
Around a year and a half after Ainsley’s nerve transfer surgeries, her hand closing was improved and strong in the left, but her right hand produced only a flicker of movement. With her surgeons, it was determined that her right hand was not improving further so Ainsley went ahead with Plan B – a tendon transfer for the thumb to index finger pinch grip. Ainsley describes the tendon transfer recovery as “hard” compared to nerve transfer because “I was in a cast and not allowed to move for 6 weeks”. In contrast, she was able to move around immediately after nerve transfer surgery with pain medications. That said, the tendon transfer was a success!
Where they are at today
 Ainsley is now 2 years after the nerve transfers and has gained the ability to fully open both hands. On the left, her restored hand closing from nerve transfer is very strong and she can pick things up. The right-hand pinch gained from the tendon transfer is functional and continues to build strength. All these improvements in her fingers and hands mean that Ainsley can use her cell phone with finger gestures, scratch an itch, adjust her hair, and hold and use things like cutlery, a toothbrush, makeup, and bank cards. The triceps nerve transfer has recovered to the point where she can now extend both arms against gravity. These days, Ainsley is getting ready to hit the road in a custom hand control vehicle, something that would not have been possible if not for the triceps surgeries that improved her strength enough to turn a steering wheel. Hopeful for the future, Ainsley says that she is “still improving everyday”.
Ainsley is now 2 years after the nerve transfers and has gained the ability to fully open both hands. On the left, her restored hand closing from nerve transfer is very strong and she can pick things up. The right-hand pinch gained from the tendon transfer is functional and continues to build strength. All these improvements in her fingers and hands mean that Ainsley can use her cell phone with finger gestures, scratch an itch, adjust her hair, and hold and use things like cutlery, a toothbrush, makeup, and bank cards. The triceps nerve transfer has recovered to the point where she can now extend both arms against gravity. These days, Ainsley is getting ready to hit the road in a custom hand control vehicle, something that would not have been possible if not for the triceps surgeries that improved her strength enough to turn a steering wheel. Hopeful for the future, Ainsley says that she is “still improving everyday”.
 Dan is coming up on 3 years after the nerve transfers. Although his grip is not strong, it is strong enough that he can use and squeeze the brakes on the new e-bike attachment for his wheelchair, which he would not have been able to do without the nerve transfer. Being able to extend his fingers has made it much easier to open his hand to grasp things and move them around. He has more function in his hands then before, but he still has not recovered some of the strength he lost after the surgeries. Dan described how “Before the surgery I could lift a full backpack of groceries off of the back of my chair now I have difficulty if there’s any weight in my bag.” That said, he is still waiting to see how much he improves, explaining, “…it’s coming, it’s just not there yet. I think they say the plateau is four years for this surgery…”, referencing experts who say that improvements for nerve transfers typically reach their peak at around 4 years.
Dan is coming up on 3 years after the nerve transfers. Although his grip is not strong, it is strong enough that he can use and squeeze the brakes on the new e-bike attachment for his wheelchair, which he would not have been able to do without the nerve transfer. Being able to extend his fingers has made it much easier to open his hand to grasp things and move them around. He has more function in his hands then before, but he still has not recovered some of the strength he lost after the surgeries. Dan described how “Before the surgery I could lift a full backpack of groceries off of the back of my chair now I have difficulty if there’s any weight in my bag.” That said, he is still waiting to see how much he improves, explaining, “…it’s coming, it’s just not there yet. I think they say the plateau is four years for this surgery…”, referencing experts who say that improvements for nerve transfers typically reach their peak at around 4 years.
 Even though Caleb is only 1 year and 3 months after the nerve transfer and still has a long way to go, he is already happy with the improvements. “Going from zero movement in my fingers to now, it’s kind of huge”. The first big impact the nerve transfers had in Caleb’s day-to-day life was probably around four months in, when he was able to open his hand to grab his toothbrush without any kind of assistance. He can now grab a toothbrush or pop can and hold on to it without a problem. His triceps progress has been harder to pin down. There is some movement in his left arm and a small amount in his right arm but he wonders if that would have come back naturally after SCI regardless of the nerve transfers. Whether or not the improvements came from the nerve transfers or from natural recovery, it has been a big help for Caleb’s mobility and being able to shift and transfer.
Even though Caleb is only 1 year and 3 months after the nerve transfer and still has a long way to go, he is already happy with the improvements. “Going from zero movement in my fingers to now, it’s kind of huge”. The first big impact the nerve transfers had in Caleb’s day-to-day life was probably around four months in, when he was able to open his hand to grab his toothbrush without any kind of assistance. He can now grab a toothbrush or pop can and hold on to it without a problem. His triceps progress has been harder to pin down. There is some movement in his left arm and a small amount in his right arm but he wonders if that would have come back naturally after SCI regardless of the nerve transfers. Whether or not the improvements came from the nerve transfers or from natural recovery, it has been a big help for Caleb’s mobility and being able to shift and transfer.
It is clear that the functions gained and the rate of recovery for nerve transfer surgeries can vary widely. However, what determines the success and speed of recovery after surgery is still an area of active research.
Advice and recommendations
There is a sense of excitement about nerve transfer surgeries and their potential for helping patients with SCI. The procedure has had many successes but so much research remains to be done to improve outcomes. Reflecting on their own journeys, Ainsley, Dan, and Caleb offered some words of advice on nerve transfers for both the clinicians who make them happen and the people who will need them in the future.
Ainsley encourages others to advocate for their treatment options. She and her family found a specific nerve transfer that they believed would be a good option for her, and worked closely with the surgical team. The results were good and Ainsley tells us that since then, that surgical team has had many successes with that same procedure on other people. Overall Ainsley believes that the surgery was “very much worth it. The benefits outweigh the cons, and I was very lucky that I had great surgeons”. Ainsley and her dad also strongly recommend considering tendon transfers as a backup for nerve transfers.
Dan offered some words of caution as a nerve transfer recipient with chronic SCI. He felt like he went into the surgery with rose-coloured glasses on, only to discover that the recovery was not seamless and there were many unforeseen obstacles. Having lived with an SCI for many years, Dan says, “…there were so many things that I had learned how to do in those five years that all of a sudden, I wasn’t able to do.” and he thinks the doctors did not realize these adaptations would be impacted by the surgery. He had the impression that he would “be able to do everything you could do before”, but in reality, he lost some abilities for a while, including being able to transfer and use a manual wheelchair.
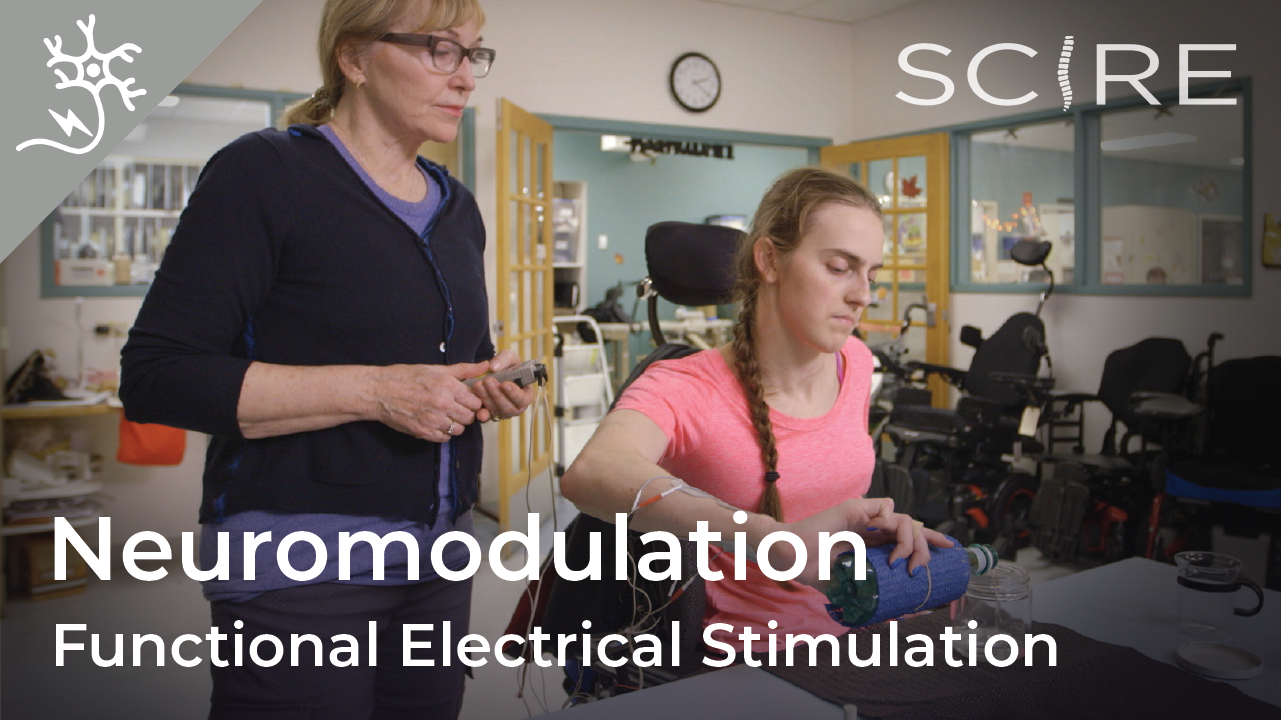
Considering his rehabilitation, Dan wonders if more could be done at home. For example, he heard of other people who did a lot of functional electrical stimulation (FES) for their nerve transfer rehabilitation and proposed, “You can set up somebody to do FES by themselves on their arms, right?… The client can be shown how to do it… and do it at home.” On the other hand, Ainsley had the chance to try FES but found that even when working with an occupational therapist, it was too difficult to correctly place the electrodes. That said, rehabilitation is specific to the individual and what doesn’t work for one person might work for another!
Dan also suggested that rehabilitation after surgery could be more structured, “like a program that you do after the surgery, then for the next three months after”.
Having spoken with Dan about this before, Caleb agrees that following a program would be useful. He thinks his occupational and physical therapy team did a great job but a clear step-by-step handbook or video outlining the exercises and the braces used would be nice. While Caleb did exercises in rehabilitation, some videos of him were recorded for him to refer back to but he thinks it would also be beneficial to see someone demonstrate the exercises, like a guide.
When asked what he would say to someone considering nerve transfer, Caleb admits, “Because mine went so well, when I talk to people, I’m like yeah it went really good. It’s gonna be all benefit…”. Still, he recognizes that it does not go that well for everyone, adding that “it would be awesome to have this larger compilation of all the things that went well and didn’t go well (for different people), so that way, people could really see the options…”
All in all, we can see that every experience with nerve transfer surgery will be different; every person will encounter unique obstacles, surprises, and benefits. Even with all the research papers and educational resources, nothing can portray an experience in full colour quite like a conversation with a person who has been through it.
Videos of Ainsley, Dan, and Caleb demonstrating some of the movements and functions they have gained in rehabilitation after nerve transfer surgery.
![]()






 Age: 37
Age: 37
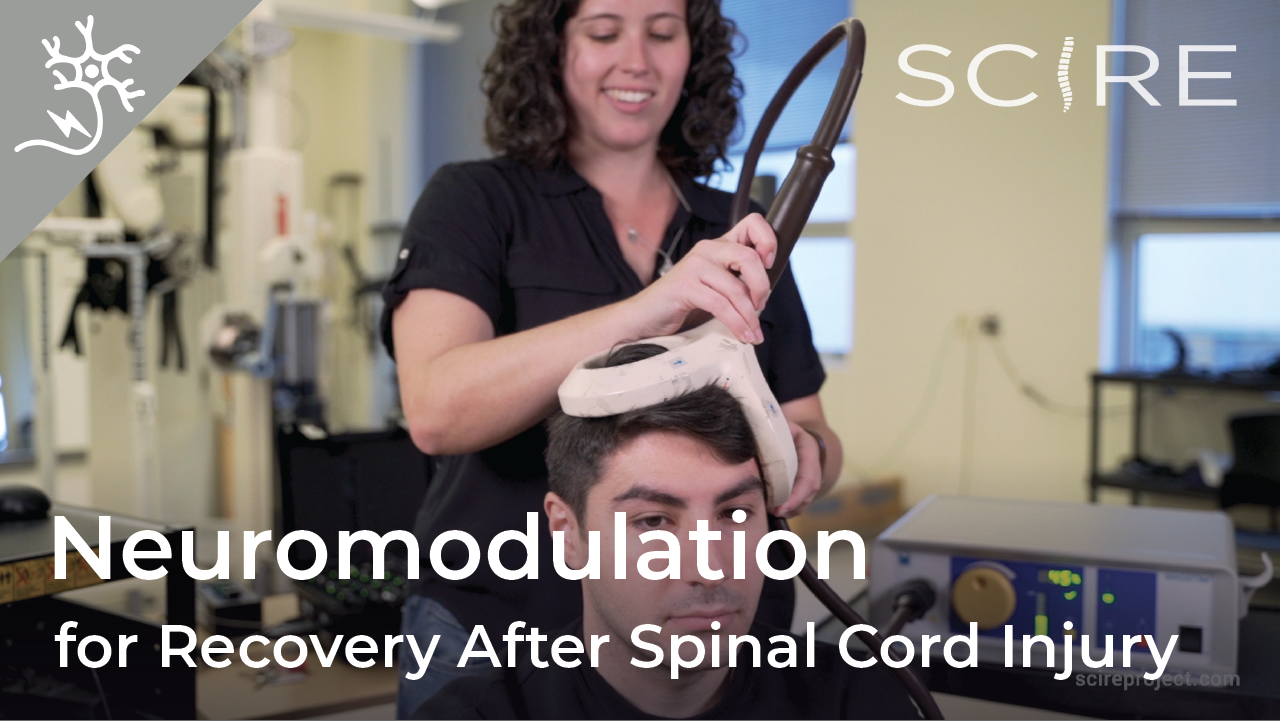

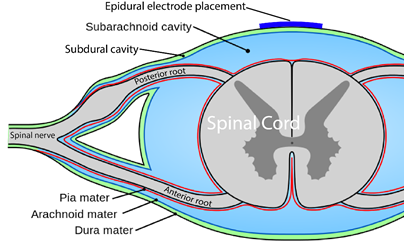
 You are probably familiar with the term “epidural” already, as it is often mentioned in relation to childbirth. If a new mother says she had an epidural, what she usually means is that she had pain medication injected into the epidural space for the purpose of managing pain during birth.
You are probably familiar with the term “epidural” already, as it is often mentioned in relation to childbirth. If a new mother says she had an epidural, what she usually means is that she had pain medication injected into the epidural space for the purpose of managing pain during birth.
 Neuromodulation methods to manage bladder function have usually involved stimulation of the sacral nerves (which are outside of the spinal cord), not with epidural spinal cord stimulation. This is reflected in the fact that almost no research exists regarding the effects of epidural stimulation on bowel and bladder function in the previous century.
Neuromodulation methods to manage bladder function have usually involved stimulation of the sacral nerves (which are outside of the spinal cord), not with epidural spinal cord stimulation. This is reflected in the fact that almost no research exists regarding the effects of epidural stimulation on bowel and bladder function in the previous century.
 One of the consequences of SCI is the loss of muscle mass below the injury and a tendency to accumulate fat inside the abdomen (abdominal fat or visceral fat) and under the skin (subcutaneous fat). These changes and lower physical activity after SCI increase the risk for several diseases.
One of the consequences of SCI is the loss of muscle mass below the injury and a tendency to accumulate fat inside the abdomen (abdominal fat or visceral fat) and under the skin (subcutaneous fat). These changes and lower physical activity after SCI increase the risk for several diseases. The first use of epidural stimulation was as a treatment for chronic pain in the 1960s. Since then, it has been widely used for chronic pain management in persons without SCI. However, it is important to recognize that the chronic pain experienced by those without SCI is different from the chronic neuropathic pain experienced after SCI. This may explain, to some extent, why epidural stimulation has not been as successful in pain treatment for SCI. The mechanism by which electrical stimulation of the spinal cord can help with pain relief is unclear. Some research suggests that special nerve cells that block pain signals to the brain may be activated by epidural stimulation.
The first use of epidural stimulation was as a treatment for chronic pain in the 1960s. Since then, it has been widely used for chronic pain management in persons without SCI. However, it is important to recognize that the chronic pain experienced by those without SCI is different from the chronic neuropathic pain experienced after SCI. This may explain, to some extent, why epidural stimulation has not been as successful in pain treatment for SCI. The mechanism by which electrical stimulation of the spinal cord can help with pain relief is unclear. Some research suggests that special nerve cells that block pain signals to the brain may be activated by epidural stimulation. Using epidural stimulation to improve respiratory function is useful because it contracts the diaphragm and other muscles that help with breathing. Also, these muscles are stimulated in a way that imitates a natural pattern of breathing, reducing muscle fatigue. More common methods of improving respiratory function do not use epidural stimulation, but rather, directly stimulate the nerves that innervate the respiratory muscles. While such methods significantly improve quality of life and function in numerous ways, they are not without issues, including muscle fatigue from directly stimulating the nerves.
Using epidural stimulation to improve respiratory function is useful because it contracts the diaphragm and other muscles that help with breathing. Also, these muscles are stimulated in a way that imitates a natural pattern of breathing, reducing muscle fatigue. More common methods of improving respiratory function do not use epidural stimulation, but rather, directly stimulate the nerves that innervate the respiratory muscles. While such methods significantly improve quality of life and function in numerous ways, they are not without issues, including muscle fatigue from directly stimulating the nerves. In another study with two middle-aged females 5-10 years post-injury, one reported no change in sexual function and the other reported the ability to experience orgasms with epidural stimulation, which was not possible since her injury.
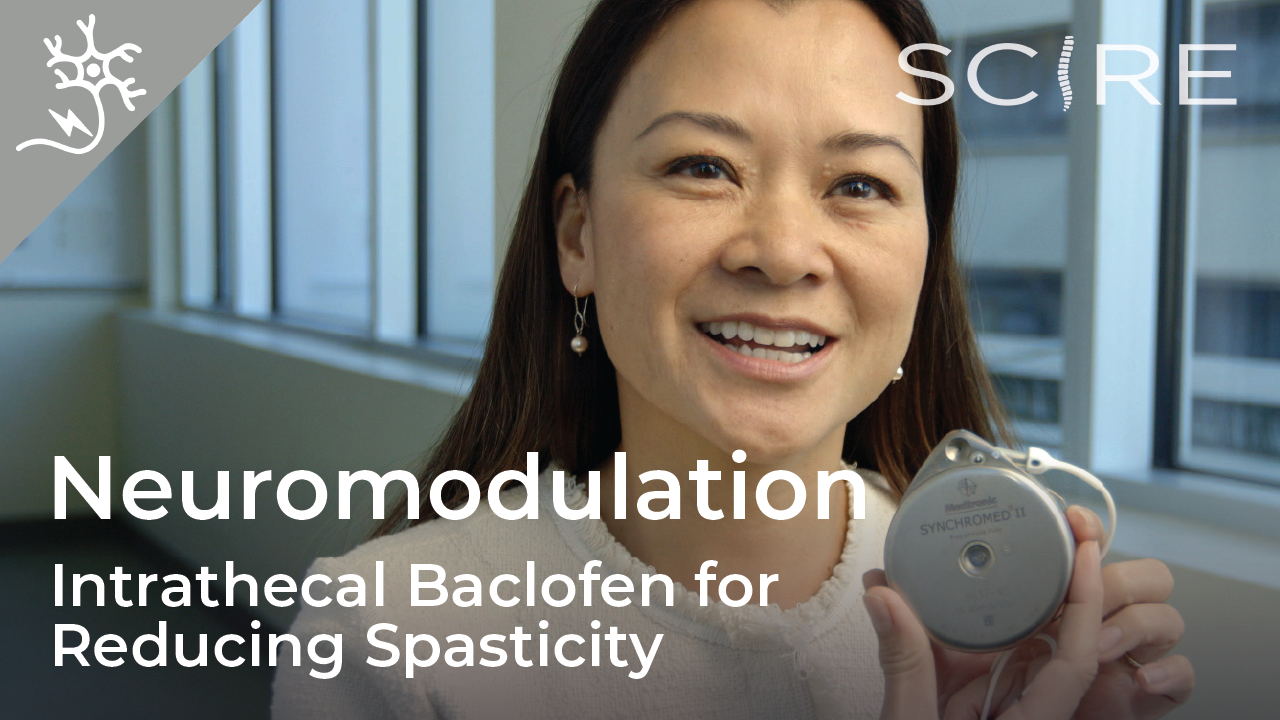
In another study with two middle-aged females 5-10 years post-injury, one reported no change in sexual function and the other reported the ability to experience orgasms with epidural stimulation, which was not possible since her injury. Botulinum toxin (Botox) injections and surgically implanted intrathecal Baclofen pumps are the most common ways to manage spasticity. Baclofen pumps are not without issues, however. Many individuals do not qualify for this treatment if they have seizures or blood pressure instability, and pumps require regular refilling.
Botulinum toxin (Botox) injections and surgically implanted intrathecal Baclofen pumps are the most common ways to manage spasticity. Baclofen pumps are not without issues, however. Many individuals do not qualify for this treatment if they have seizures or blood pressure instability, and pumps require regular refilling. In a study with a single participant (weak evidence) investigating walking, an individual implanted with an epidural stimulator also reported improvement in body temperature control, however details were not provided. More research is required to understand the role of epidural stimulation for temperature regulation.
In a study with a single participant (weak evidence) investigating walking, an individual implanted with an epidural stimulator also reported improvement in body temperature control, however details were not provided. More research is required to understand the role of epidural stimulation for temperature regulation.
 In severe SCI, individuals may suffer from chronic low blood pressure and orthostatic hypotension (fall in blood pressure when moving to more upright postures). These conditions can have significant effects on health and quality of life. Some recent studies have looked at how epidural stimulation affects cardiovascular function to improve orthostatic hypotension. Overall, they show (weak evidence) that epidural stimulation immediately increases blood pressure in individuals with low blood pressure while not affecting those who have normal blood pressure. They also showed that there is a training effect with repeated stimulation. This means that after consistently using stimulation for a while, normal blood pressure can occur even without stimulation when moving from lying to sitting.
In severe SCI, individuals may suffer from chronic low blood pressure and orthostatic hypotension (fall in blood pressure when moving to more upright postures). These conditions can have significant effects on health and quality of life. Some recent studies have looked at how epidural stimulation affects cardiovascular function to improve orthostatic hypotension. Overall, they show (weak evidence) that epidural stimulation immediately increases blood pressure in individuals with low blood pressure while not affecting those who have normal blood pressure. They also showed that there is a training effect with repeated stimulation. This means that after consistently using stimulation for a while, normal blood pressure can occur even without stimulation when moving from lying to sitting. For individuals with tetraplegia, even some recovery of hand function can mean a big improvement in quality of life. Research into using epidural stimulation to improve hand function consists of one case study (weak evidence) involving two young adult males who sustained motor complete cervical spinal cord injury over 18 months prior.
For individuals with tetraplegia, even some recovery of hand function can mean a big improvement in quality of life. Research into using epidural stimulation to improve hand function consists of one case study (weak evidence) involving two young adult males who sustained motor complete cervical spinal cord injury over 18 months prior. Being able to control your trunk (or torso) is important for performing everyday activities such as picking things up or reaching for items. One study found that using epidural stimulation can increase the amount of distance you are able to lean forward. The improvement in forward reach occurred immediately when the stimulation was turned on. The two participants in this study were also able to reach more side to side as well, but the improvement was minor.
Being able to control your trunk (or torso) is important for performing everyday activities such as picking things up or reaching for items. One study found that using epidural stimulation can increase the amount of distance you are able to lean forward. The improvement in forward reach occurred immediately when the stimulation was turned on. The two participants in this study were also able to reach more side to side as well, but the improvement was minor.
 Some studies have also found that with extensive practice (e.g., 80 sessions), independent standing (i.e., standing without the help of another person, but holding onto a bar) may be achieved without epidural stimulation. Gaining the ability to stand may also occur with stand training combined with epidural stimulation. However, the findings with regard to the effect of stand training with epidural stimulation have been mixed. For example, one study showed that stand training for 5 days a week over a 4 month period with epidural stimulation resulted in independent standing for up to 10 minutes in an individual with a complete C7 injury, while another study has suggested that independent standing for 1.5 minutes can be achieved with epidural stimulation and 2 weeks of non-step specific training in an individual with complete T6 injury.
Some studies have also found that with extensive practice (e.g., 80 sessions), independent standing (i.e., standing without the help of another person, but holding onto a bar) may be achieved without epidural stimulation. Gaining the ability to stand may also occur with stand training combined with epidural stimulation. However, the findings with regard to the effect of stand training with epidural stimulation have been mixed. For example, one study showed that stand training for 5 days a week over a 4 month period with epidural stimulation resulted in independent standing for up to 10 minutes in an individual with a complete C7 injury, while another study has suggested that independent standing for 1.5 minutes can be achieved with epidural stimulation and 2 weeks of non-step specific training in an individual with complete T6 injury. Earlier research has found that epidural stimulation can help with the development of walking-like movements, but these movements do not resemble “normal” walking. Instead, they resemble slight up and down movements of the leg. Recent studies have shown that with 10 months of practicing activities while lying down on the back and on the side, in addition to standing and stepping training, people are able to take a step without assistance from another person or body weight support. While some individuals in these studies have been able to regain some walking function, they are walking at a very slow pace, ranging from 0.19 meters per second to 0.22 meters per second. This is much slower than the 0.66 meters per second required for community walking. For example, of the 4 participants in one study, two were able to walk on the ground with a walker, one was only able to walk on a treadmill, and one was able to walk on the ground while holding the hands of another person. These differences in walking abilities gained by participants were not expected.
Earlier research has found that epidural stimulation can help with the development of walking-like movements, but these movements do not resemble “normal” walking. Instead, they resemble slight up and down movements of the leg. Recent studies have shown that with 10 months of practicing activities while lying down on the back and on the side, in addition to standing and stepping training, people are able to take a step without assistance from another person or body weight support. While some individuals in these studies have been able to regain some walking function, they are walking at a very slow pace, ranging from 0.19 meters per second to 0.22 meters per second. This is much slower than the 0.66 meters per second required for community walking. For example, of the 4 participants in one study, two were able to walk on the ground with a walker, one was only able to walk on a treadmill, and one was able to walk on the ground while holding the hands of another person. These differences in walking abilities gained by participants were not expected.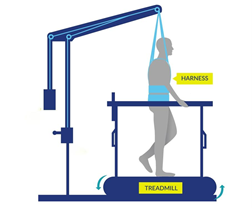
 In Canada, the cost for an institution to install an epidural stimulation system for back pain in those without spinal cord injury, which is a common procedure, was $21,595 CAD. The cost incurred by a Canadian citizen undergoing implantation in Canada is $0 as it is covered by publicly funded health care.
In Canada, the cost for an institution to install an epidural stimulation system for back pain in those without spinal cord injury, which is a common procedure, was $21,595 CAD. The cost incurred by a Canadian citizen undergoing implantation in Canada is $0 as it is covered by publicly funded health care.