Author: SCIRE Community Team | Reviewer: Chris S. Bailey | Published: 22 January 2018 | Updated: ~
Neuroprotection describes a wide range of treatments that aim to protect the spinal cord from further damage in the hours to weeks after an injury first happens. This page explains what neuroprotection is and outlines what the most promising treatments are right now.
Key Points
- Spinal cord damage can worsen in the days to weeks after a spinal cord injury (SCI) because of processes like inflammation and lack of blood flow. This is called secondary injury.
- Neuroprotection is the use of medical treatments early after the initial injury to help protect the spinal cord from further damage.
- A wide range of medications has been suggested as neuroprotection, including the steroid methylprednisolone. Other types of treatments, such as cooling the body, have also been studied.
- Neuroprotection treatments are controversial at this time because there is not enough evidence to support using most treatments and there is disagreement amongst experts about how to interpret research findings for using methylprednisolone.
- Neuroprotection is an emerging area of research and many current studies are ongoing.
Neuroprotection is the use of medical treatments to protect the spinal cord from further injury in the hours to weeks after a spinal cord injury first happens. The term neuroprotection is used to describe a wide range of mainly experimental treatments that aim to reduce damage known as secondary injury.
Secondary injury
There are two main phases of damage that happen after a spinal cord injury, primary injury and secondary injury. The damage that is directly caused by the initial injury is called primary injury. Primary injury happens when the spinal cord is bruised, compressed, pulled or torn, which directly injures the nerve cells and spinal cord tissues.
As the body responds to the initial injury, several bodily processes can happen, which further damage the spinal cord. This is known as secondary injury. Secondary injury is caused by several processes, such as inflammation, lack of blood flow (ischemia), and build-up of damaging chemicals, which can worsen the original injury.
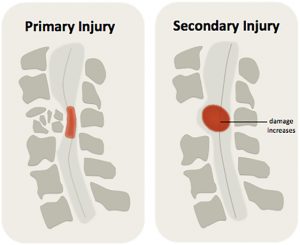
Secondary injury can worsen the spinal cord injury, expanding the size of the injury and leading to further loss of function.1
Secondary injury processes are responsible for expanding the size of the injury in the days to weeks afterwards and causing further loss of function. They can also make it harder for the body to heal.
Neuroprotection is intended to help protect the spinal cord from further damage, it is not intended to heal or repair already damaged tissues (this is called regeneration). Neuroprotective treatments are usually given as early as possible after injury, which is usually within the first 24 hours after injury or at the time of the first surgery.
Neuroprotection is also used to help protect against further nerve damage in other neurological conditions, such as brain injuries and degenerative diseases like amyotrophic lateral sclerosis (ALS).
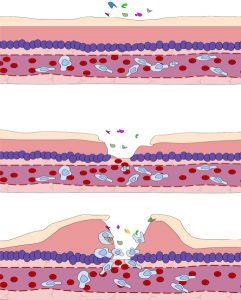
During inflammation, special immune cells help get rid of foreign substances. This can lead to localized swelling.2
Inflammation and swelling
Inflammation is a natural bodily process where cells from the immune system (such as white blood cells) are brought to the site of an injury or illness. These cells remove harmful bacteria and dead cells from the area to help with healing.
Some inflammation is needed for healing. However, when it is excessive or continues for a long time, it can be damaging. Inflammation can end up breaking down healthy nerve cells (neurons), which makes the injury worse. Inflammation and other factors can also contribute to swelling of the spinal cord near the injury. Swelling can compress the spinal cord even more, damaging nerve cells and impairing blood flow.
Lack of blood flow
When the spinal cord is injured, the small blood vessels that provide oxygen and nutrients to the cord are also injured. If the blood vessels are torn, they may bleed within the spinal cord causing a bruise (called haemorrhage), which can increase the pressure on the spinal tissues and cause damage.
If the blood vessels are compressed or if there is not enough whole body blood pressure (which happens as a part of neurogenic shock), they are also unable to maintain adequate blood flow to the cord. This is called ischemia (pronounced ‘iss-kee-mee-ah’). A lack of blood flow means that oxygen or nutrients cannot reach the tissues, which can damage or kill healthy cells. Ischemia can become severe within hours after the injury and can damage nerve cells and surrounding tissues, causing the injury to get bigger.
Build-up of damaging chemicals
Damaged nerve cells can cause the release of chemical compounds like glutamate. Glutamate is a neurotransmitter that stimulates nerve cells to fire. However, too much glutamate can overstimulate the cells, causing calcium to build up inside them, which damages and kills healthy cells. This is called excitotoxicity. Excitotoxicity is a major cause of secondary damage after SCI.
Inflammation, excitotoxicity and cell damage can release by-products called free radicals. Free radicals are molecules that are unstable and highly reactive. Free radicals can damage DNA, the fragile outer membranes, and other parts of cells.
Nerve cell death
Cells can die because of damage or injury (called necrosis) or because the body triggers the cells to self-destruct through a process called apoptosis.
Apoptosis is sometimes called ‘programmed cell death’ because it is a natural part of how the body gets rid of cells it doesn’t need.
Under normal circumstances, apoptosis is carefully controlled by the body. However, certain conditions can trigger apoptosis when it is not needed. For example, damage to parts of the cell or chemical imbalances around or within the cell can trigger apoptosis.
After an SCI, secondary injury processes like excitotoxicity, inflammation, and the release of free radicals can trigger apoptosis. This can affect both nerve cells (neurons) and supporting cells like the cells that maintain myelin. This can even happen far away from the injury. Cell apoptosis after SCI expands the area of damage by killing previously healthy neurons and supporting cells.
‘Neuroprotection’ refers to a wide range of different treatments, including medications and other treatments like cooling the body that aim to reduce secondary injury. It is sometimes used to describe surgical decompression and blood pressure control after SCI, although these treatments are not discussed on this page.
While there are many different neuroprotective treatment options being explored, below we briefly outline the most promising treatments currently being studied.
Most neuroprotection treatments are experimental
Currently, there are no widely accepted treatments used for neuroprotection. With the exception of methylprednisolone (see below), most of the potential treatments are currently being tested in research studies. Because of the experimental nature of these treatments, most of the treatments outlined below are not available for most people or used outside of research settings.
For details about the progress of ongoing clinical trials, please visit ClinicalTrials.gov.
Steroids (Methylprednisolone)
The steroid medication Methylprednisolone is the most well-known neuroprotective treatment. It is also the only treatment that is currently used outside of research studies. High doses of methylprednisolone may affect many different aspects of secondary injury, including reducing inflammation and damage from free radicals that are thought to help prevent cells from dying after injury.
Three large-scale clinical trials were completed during 1980’s and 1990’s, which established methylprednisolone as a standard treatment that all patients with SCI should receive. However, later debate among experts criticized conclusions made in these studies and questioned the value of methylprednisolone as a treatment. It has been argued that methylprednisolone has shown only limited benefits for recovery, but well-established risk of side effects like infections.
At this time, methylprednisolone continues to be a controversial treatment among experts. It may be used in some clinical settings in certain groups of people with SCI.
Riluzole
Riluzole is a drug that blocks sodium from entering into nerve cells, which may help to reduce excitotoxicity. Riluzole is currently used to treat the degenerative neurological condition amyotrophic lateral sclerosis (ALS).
Animal studies have shown that Riluzole is effective as a neuroprotection treatment in rats with SCI. One preliminary study supported that Riluzole is safe and has potential to provide neuroprotection after SCI in humans. Currently, a large-scale clinical trial is in progress to determine whether Riluzole is effective as neuroprotection after SCI.
Minocycline
Minocycline is an antibiotic medication that is most commonly used to treat bacterial infections. Minocycline may also have neuroprotective effects after SCI. Animal studies have shown that minocycline may suppress immune cells involved in inflammation, cell death and the release of damaging chemicals. An early clinical trial has shown that IV minocycline is safe for use after acute SCI, and has potential as neuroprotection in people with incomplete SCI. Currently, a large-scale study is ongoing to investigate whether minocycline is effective as neuroprotection after SCI.
Cooling the body (therapeutic hypothermia)
Cooling the body, known as therapeutic hypothermia, is a treatment that involves reducing the temperature of the body to help protect against further damage. The body is cooled by inserting a cooling catheter into a blood vessel, which cools blood moving through circulation by a few degrees. Reducing body temperature slows metabolism, which can reduce inflammation and minimize further damage. This procedure is currently used to help prevent neurological damage after cardiac arrest.
A small preliminary study has shown that therapeutic hypothermia is safe and has potential as neuroprotection after SCl. Currently, a clinical trial is underway to determine whether spinal cord cooling is effective as neuroprotection for SCI in humans.
Cerebrospinal fluid drainage
Cerebrospinal fluid (CSF) drainage is a technique that involves inserting a small catheter through the coverings of the spinal cord to drain a small amount of the fluid that surrounds the spinal cord and brain (called cerebrospinal fluid). Draining the cerebrospinal fluid is thought to help relieve pressure and reduce further injury to the spinal cord.
Animal studies have shown that cerebrospinal fluid drainage may help improve spinal cord blood flow when combined with careful blood pressure management. One small research study has shown that cerebrospinal fluid drainage was safe for use in people with acute SCI. A larger study has been completed to determine whether cerebrospinal fluid drainage is effective as neuroprotection after SCI. However, the results have not yet been published.
Magnesium
Magnesium has been proposed as a neuroprotective treatment because it can help to reduce excitotoxicity and inflammation. Animal studies have shown benefits for magnesium as neuroprotection.
Cethrin (VX-210)
Cethrin (VX-210) is a medication that reduces the effects of Rho, a protein that is present in inflammation. Rho causes damage to nerve cells and prevents nerve cell regrowth. Cethrin reverses the action of Rho, which may help protect nerve cells and allow neurons to regrow. An early study has shown that Cethrin is safe. A clinical trial testing whether it is effective has been completed with the results pending publication.
Other treatments being studied
A wide range of other treatments have been or are currently being studied. These include:
- Fibroblast growth factor
- Hepatocyte growth factor
- Erythropoetin (EPO)
- GM-1 Ganglioside (Sygen)
- Granulocyte-colony stimulating factor (G-CSF)
- Thyrotropin-releasing hormone (TRH)
- Glibenclamide or glyburide
- Special diets such as intermittent fasting
Neuroprotective treatments that are not effective
Research evidence on the following treatments has suggested that they are not effective for use as neuroprotection after SCI. These include:
- Naloxone
- Tirilazad mesylate (was not more effective than methylprednisolone, but had more side effects)
- Nimodipine
- Gacyclidine
Secondary injury is a complicated process that scientists are still working to understand. While there are many promising treatments currently being studied, there are no widely accepted treatments so far. Neuroprotection is a relatively new area of study compared to other aspects of medicine and there are a number of other factors that makes neuroprotection difficult to study.
Testing experimental treatments is lengthy and expensive
 Most neuroprotective treatments are experimental and have not been tested in humans. This means that each treatment must undergo vigorous testing to determine if it is safe and effective. There are several phases of study that must be done. Usually, research studies are tested on animals first, and then typically go through at least three phases of human studies (Phase I, II, and III clinical trials) before a treatment can be determined to be safe and effective for real world use. These trials can cost millions of dollars and take several years to complete.
Most neuroprotective treatments are experimental and have not been tested in humans. This means that each treatment must undergo vigorous testing to determine if it is safe and effective. There are several phases of study that must be done. Usually, research studies are tested on animals first, and then typically go through at least three phases of human studies (Phase I, II, and III clinical trials) before a treatment can be determined to be safe and effective for real world use. These trials can cost millions of dollars and take several years to complete.
Designing and conducting high quality studies is very difficult
 It is also very difficult to design and carry out high quality studies. There are many factors relevant to the design and how the study findings are analyzed that can affect the study’s findings. This is why many previous research studies (such as those done on methylprednisolone), are controversial even amongst experts.
It is also very difficult to design and carry out high quality studies. There are many factors relevant to the design and how the study findings are analyzed that can affect the study’s findings. This is why many previous research studies (such as those done on methylprednisolone), are controversial even amongst experts.
In addition, neuroprotection treatments are usually given immediately after the injury during a highly stressful and confusing period. It can be challenging for patients and their families to determine whether they want to take part in a study and give their consent to participate.
Promising experimental treatments often do not translate into effective treatments
 Unfortunately, many treatments that are shown to be effective in animal studies do not go on to show the same effects in the real world scenarios involved in clinical trials. Translating research into effective treatments is a complex process with many steps that are undertaken to ensure that treatments are safe and effective.
Unfortunately, many treatments that are shown to be effective in animal studies do not go on to show the same effects in the real world scenarios involved in clinical trials. Translating research into effective treatments is a complex process with many steps that are undertaken to ensure that treatments are safe and effective.
Because secondary injury is so complex, some researchers believe that it is unlikely that one single treatment will be discovered that will completely “protect” the spinal cord from further damage. Research may lead clinicians to several different treatment options that may be used together or in different situations to help protect the spinal cord from further damage.
Neuroprotection is the use of medical treatments early after the initial injury to help protect the spinal cord from further damage caused by secondary injury.
There are many different treatments being studied for use as neuroprotection. The steroid methylprednisolone is the most well known treatment, but its use is controversial among experts. Most neuroprotective treatments are currently being investigated in research studies.
Neuroprotection after SCI is an emerging area of research and clinical care that we will learn more about as research findings arise in the next few years.
Parts of this page have been adapted from the SCIRE Project (Professional) “Neuroprotection during the Acute Phase of Spinal Cord Injury” Chapter:
Mullen E, Mirkowski M, Hsieh JTC, Bailey C, McIntyre A, Teasell RW. (2015). Neuroprotection during the Acute Phase of Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Research Evidence. Version 5.0: p 1-42.
Available from: https://scireproject.com/evidence/acute-evidence/neuroprotection-during-acute-phase-of-spinal-cord-injury/
Evidence for “Steroids (Methylprednisolone)”
Alibai E, Zand F, Rahimi A, Rezaianzadeh A. Erythropoietin plus methylprednisolone or methylprednisolone in the treatment of acute spinal cord injury: a preliminary report. Acta Med Iran 2014; 52(4):275-279.
Amar AP, Levy ML. Surgical controversies in the management of spinal cord injury. J Am Coll Surgeons 1999; 188(5):550-566.
Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the second national acute spinal cord injury study. New Engl J Med 1990; 322(20):1405-1411.
Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997; 277(20):1597-1604.
Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up: results of the third national acute spinal cord injury randomized controlled trial. J Neurosurg 1998; 89(5):699-706.
Chikuda H, Yasunaga H, Takeshita K, et al. Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg Med J 2014; 31(3):201-206.
Christie SD, Comeau B, Myers T, Sadi D, Purdy M, Mendez I. Duration of lipid peroxidation after acute spinal cord injury in rats and the effect of methylprednisolone: laboratory investigation. Neurosurg Focus 2008; 25(5).
George ER, Scholten DJ, Buechler CM, et al. Failure of methylprednisolone to improve the outcome of spinal cord injuries. Am Surgeon 1995; 61(8):659-664.
Gerhart KA, Johnson RL, Menconi J, Hoffman RE, Lammertse DP. Utilization and effectiveness of methylprednisolone in a population-based sample of spinal cord injured persons. Paraplegia 1995; 33(6):316-321.
Gerndt SJ, Rodriguez JL, Pawlik JW, et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma 1997; 42(2):279-284.
Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg 1992; 76(1):13-22.
Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine 2009; 34(20):2121-2124.
Lee BH, Lee KH, Yoon DH, et al. Effects of methylprednisolone on the neural conduction of the motor evoked potentials in spinal cord injured rats. J Korean Med Sci 2005; 20(1):132-138.
Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine 2001; 26(4):426-430.
Prendergast MR, Saxe JM, Ledgerwood AM, et al. Massive steroids do not reduce the zone of injury after penetrating spinal cord injury. J Traum 1994; 37(4):576-580.
Rasool T, Wani MA, Kirmani AR, et al. Role of methylprednisolone in acute cervical cord injuries. Indian J Surg 2004; 66(3):156-159.
Suberviola B, González-Castro A, Llorca J, Ortiz-Melón F, Miñambres E. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury 2008; 39(7):748-752.
Vaquero J, Zurita M, Oya S, Aguayo C, Bonilla C. Early administration of methylprednisolone decreases apoptotic cell death after spinal cord injury. Histol Histopathol 2006; 21(10-12):1091-1102.
Xiong M, Chen S, Yu H, Liu Z, Zeng Y, Li F. Neuroprotection of erythropoietin and methylprednisolone against spinal cord ischemia-reperfusion injury. J Huazhong Univ Sci 2011; 31(5):652-656.
Zhuang C, Wang L, Xu Y. Early methylprednisolone impact treatment for sensory and motor function recovery in patients with acute spinal cord injury: a self-control study. Neural Regen Res 2008; 3(5):577-580.
Evidence for “Riluzole”
Fehlings MG, Nakashima H, Nagoshi N, Chow DS, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016 Jan;54(1):8-15.
Grossman RG, Fehlings MG, Frankowski RF, et al. A prospective, multicenter, Phase i matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotraum 2014; 31(3):239-255.
Satkunendrarajah K, Nassiri F, Karadimas SK, Lip A, Yao G, Fehlings MG. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol. 2016 Feb;276:59-71.
Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg 2001; 94(2 Suppl):245-256.
Wilson JR, Fehlings MG. Riluzole for acute traumatic spinal cord injury: a promising neuroprotective treatment strategy. World Neurosurg 2014; 81(5-6):825-829.
Evidence for “Minocycline”
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012; 135(Pt 4):1224-1236.
Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol 2004; 3(12):744-751.
Evidence for “Cooling the body (therapeutic hypothermia)”
Kwon BK, Mann C, Sohn HM et al. , Hypothermia for spinal cord injury. Spine J. 2008, 8, 6:859–874.
Lo TP Jr, Cho KS, Garg MSet al. , Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol . 2009, 514, 5:433–448.
Levi AD, Green BA, Wang MYet al. , Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma . 2009, 26, 3:407–415.
Evidence for “Cerebrospinal fluid drainage”
Kwon BK, Curt A, Belanger LMet al. , Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. Journal Neurosurg Spine . 2009, 10, 3:181–193.
Martirosyan NL, Kalani MY, Bichard WD, et al. Cerebrospinal fluid drainage and induced hypertension improve spinal cord perfusion after acute spinal cord injury in pigs. Neurosurgery 2015;76(4):461-469
Evidence for “Magnesium”
Kaptanoglu E, Beskonakli E, Okutan O, Selcuk Surucu H, Taskin Y. Effect of magnesium sulphate in experimental spinal cord injury: evaluation with ultrastructural findings and early clinical results. J Clin Neurosci 2003a;10(3): 329-334.
Kaptanoglu E, Beskonakli E, Solaroglu I, Kilinc A, Taskin Y. Magnesium sulfate treatment in experimental spinal cord injury: emphasis on vascular changes and early clinical results. Neurosurg Rev 2003b;26(4):283-287.
Evidence for “Cethrin (VX-210)”
Fehlings MG, Theodore N, Harrop J, et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotraum 2011; 28(5):787-796.
McKerracher L, Guertin P. Rho as a target to promote repair: translation to clinical studies with cethrin. Curr Pharm Design 2013; 19(24):4400-4410.
Evidence for “Other treatments”
Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen® multicenter acute spinal cord injury study. Spine 2001c; 26(24 Suppl):S87-S100.
Geisler FH, Dorsey FC, Coleman WP. GM-1 ganglioside in human spinal cord injury. J Neurotraum 1992; (9 Suppl 2):S517-530.
Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury – a randomized, placebo-controlled trial with GM-1 ganglioside. New Engl J Med 1991; 324(26):1829-1838.
Kamiya K, Koda M, Furuya T, et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: a comparison with high-dose methylprednisolone as a historical control. Eur Spine J 2014; 24(5):963-967.
Khorasanizadeh M, Eskian M, Vaccaro AR, Rahimi-Movaghar V. Granulocyte Colony-Stimulating Factor (G-CSF) for the Treatment of Spinal Cord Injury. CNS Drugs. 2017 Nov;31(11):911-937.
Kitamura K, Fujiyoshi K, Yamane J, et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One 2011b;6:e27706.
Kitamura K, Iwanami A, Fujiyoshi K, et al. Recombinant human hepatocyte growth factor promotes functional recovery after spinal cord injury. PLoS One 2011a;6(11):e27706.
Kitamura K, Iwanami A, Nakamura M, et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res 2007;85(11):2332-2342.
Pitts LH, Ross A, Chase GA, Faden AI. Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotraum 1995; 12(3):235-243.
Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle 2005; 4(12):1753-1757.
Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA 2001; 98(7):4044-4049.
Tadie M, Gaviria M, Mathe J, et al. Early care and treatment with a neuroprotective drug, gacyclidine, in patients with acute spinal cord injury. Rachis 2003; 15:363-376.
Takahashi H, Yamazaki M, Okawa A, et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. Eur Spine J 2012; 21(12):2580-2587.
Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci 1999;19(16):7037-7047.
Evidence for “Neuroprotective treatments that are not effective”
Flamm ES, Young W, Collins WF, Piepmeier J, Clifton GL, Fischer B. A phase I trial of naloxone treatment in acute spinal cord injury. J Neurosurg 1985; 63(3):390-397.
Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the second national acute spinal cord injury study. New Engl J Med 1990; 322(20):1405-1411.
Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997; 277(20):1597-1604.
Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up: results of the third national acute spinal cord injury randomized controlled trial. J Neurosurg 1998; 89(5):699-706.
Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord, 2000;38(2):71-76.
Tadie M, Gaviria M, Mathe J, et al. Early care and treatment with a neuroprotective drug, gacyclidine, in patients with acute spinal cord injury. Rachis, 2003;15:363-376.
Other references
Ahuja CS, Martin AR, Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016 May 27;5. pii: F1000 Faculty Rev-1017.
Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord 2015; 53(1):14-18.
Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 2001; 103(1):203-218.
Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem 2007; 14(11):1189-1197.
Fehlings MG, Baptiste DC. Current status of clinical trials for acute spinal cord injury. Injury 2005; 36(Suppl 2):SB113-SB122.
Geisler FH, Coleman WP, Grieco G, Poonian D. Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine 2001a; 26(24 Suppl):S68-S86.
Geisler FH, Coleman WP, Grieco G, Poonian D. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine 2001b; 26(24 Suppl):S58-S67.
Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004 Jan;1(1):80-100.
Hall ED. Drug development in spinal cord injury: what is the FDA looking for? J Rehabil Res Dev 2003; 40(4 Suppl 1):81-91.
Heary RF, Vaccaro AR, Mesa JJ, et al. Steroids and gunshot wounds to the spine. Neurosurgery 1997; 41(3):576-584.
Hilton BJ, Moulson AJ, Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci Lett. 2017 Jun 23;652:3-10.
Kan EM, Ling EA, Lu J. Stem cell therapy for spinal cord injury. Curr Med Chem 2010; 17(36):4492-4510.
Kavanagh RJ, Kam PCA. Lazaroids: efficacy and mechanism of action of the 21-aminosteroids in neuroprotection. Brit J Anaesth 2001; 86(1):110-119.
Kigerl K, Popovich P. Drug evaluation: ProCord – a potential cell-based therapy for spinal cord injury. IDrugs 2006; 9(5):354-360.
Levy ML, Gans W, Wijesinghe HS, SooHoo WE, Adkins RH, Stillerman CB. Use of methylprednisolone as an adjunct in the management of patients with penetrating spinal cord injury: outcome analysis. Neurosurgery 1996; 39(6):1141-1149.
Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotraum 2000; 17(10):871-890.
Merry WH, Cogbill TH, Annis BL, Lambert PJ. Functional outcome after incomplete spinal cord injuries due to blunt injury. Injury 1996; 27(1):17-20.
Meurer WJ, Barsan WG. Spinal cord injury neuroprotection and the promise of flexible adaptive clinical trials. World Neurosurg. 2014 Sep-Oct;82(3-4):e541-6.
Mocchetti I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol Life Sci 2005; 62(19-20):2283-2294.
Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000; 38(2):71-76.
Pollard ME, Apple DF. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine 2003; 28(1):33-38.
Poynton AR, O’Farrell DA, Shannon F, Murray P, McManus F, Walsh MG. An evaluation of the factors affecting neurological recovery following spinal cord injury. Injury 1997; 28(8):545-548.
Santamaria AJ, Guest JD. The current status of neuroprotection for spinal cord injury. In: Weidner N., Rupp R, Tansey K (eds) Neurological aspects of spinal cord injury. 2017 Springer, Cham.
Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res 2015;218:15-54.
Simard JM, Tsymbalyuk O, Ivanov A, et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest 2007;117(8):2105–2113.
Tator CH. Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery 1998; 42(4):696-708.
Teng YD, Mocchetti I, Wrathall JR. Basic and acidic fibroblast growth factors protect spinal motor neurones in vivo after experimental spinal cord injury. Eur J Neurosci 1998;10(2):798-802.
Wallace MC, Tator CH, Frazee P. Relationship between posttraumatic ischemia and hemorrhage in the injured rat spinal cord as shown by colloidal carbon angiography. Neurosurgery 1986; 18(4):433-439.
Waxman SG. Demyelination in spinal cord injury. J Neurol Sci 1989; 91(1-2):1-14.
Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res 2012; 37(6):1230-1244.
Image credits:
- ‘Figure 3 – There are two phases of injury after damage to the spinal cord’ from: O’Higgins M, Badner A and Fehlings M (2017) What Is Spinal Cord Injury? Front Young Minds. 5:17. doi: 10.3389/frym.2017.00017. (CC BY 3.0)
- Immune response ©Nason vassiliev, CC BY-SA 4.0
- Expensive watch ©Vectors Point, CC BY 3.0 US
- Overworked ©Luis Prado, CC BY 3.0 US
- Explosion in lab ©Gan Khoon Lay, CC BY 3.0 US





