Author: SCIRE Community Team | Reviewer: Holly Timms, Felicia Wong | Published: 21 November 2017 | Updated: ~
Spasticity is a common symptom of spinal cord injury (SCI) that causes movement problems and other symptoms. This page outlines basic information about spasticity and how it is treated after SCI.
Key Points
- Spasticity is a disorder of movement control that causes muscle spasms, increased muscle tone, and overactive reflexes. It can happen when the brain or spinal cord are damaged.
- Spasticity may cause problems with movement and posture, pain, fatigue, and many other symptoms. However, spasticity can also have benefits for movement and health.
- It is important to work together with your health team to decide whether your spasticity is problematic and worth treating.
- Treatment for spasticity usually begins with identifying specific triggers that make it worse. Management of these spasticity triggers along with other conservative treatments may alleviate these symptoms. Many of these treatments provide short-term relief of spasticity.
- Oral medications and botulinum toxin injections are also commonly used and effective for treating spasticity after SCI.

Spasticity is a movement control disorder that happens when the brain and spinal cord are damaged or do not develop properly. It is usually experienced as involuntary muscle spasms, increased muscle tone, and overactive reflexes.
Spasticity is a common symptom of SCI that can affect as many as three-quarters of people with SCI. It is more common among people with cervical and incomplete SCI. Spasticity can also be a symptom of other conditions like brain injury, stroke, and multiple sclerosis.
Spasticity can be experienced in many different ways depending on the person and the characteristics of their SCI.
Signs and symptoms of spasticity:
- Muscles that are constantly and involuntarily tensed (increased muscle tone)
- Stiff muscles that resist movement
- Muscle pain and fatigue
- Muscle spasms or jerky movements
- Uncontrolled movements or difficulty coordinating movements
- Exaggerated reflexes
- Altered posture or positioning
Spasticity is different from normal muscle tension because the amount of tension depends on the speed that the muscle is stretched. Faster movement speeds cause greater tension and resistance to movement.
Clonus
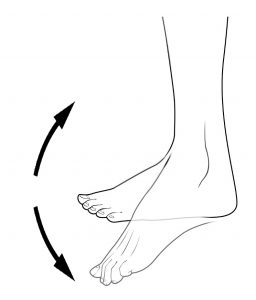
Clonus is often seen as rhythmic tapping or beating motion of the foot at the ankle.2
Clonus is a series of involuntary, rhythmic muscle contractions and relaxations, which often accompanies spasticity.
Clonus is most often seen in the ankle as a rhythmic tapping or beating motion of the foot that is triggered when there is stimulus to the ball of the foot. This can happen when putting weight onto the foot during transfers, standing, or walking. Clonus can also be experienced in other joints. Clonus can last for anywhere from a few seconds to several minutes.
Clonus is not the same as spasticity, but a related symptom that happens for similar reasons.
Spasticity may be constant or triggered by something
The symptoms of spasticity may be constant or come and go. They may also change over time. Some people will have muscle tension that is always present, while others will have spasticity that comes on or gets worse when it is triggered by something. Common spasticity triggers include:
- Movement of the arms or legs, especially quick movements
- Position changes, such as transfers, walking, or moving in bed
- Stretching
- Tight clothing or other discomfort below the level of injury
- Pressure sores, skin irritation, or wounds
- Bladder problems
- Bowel problems
- Cold temperatures
- Menstrual cycle or pregnancy
- Emotional or psychological stress
- Poor positioning in the wheelchair or bed
- Any other illnesses
A change in spasticity can be a sign of other health problems
Sudden or unexplained changes in spasticity can sometimes signal a health problem that needs attention – most commonly a bladder infection or skin breakdown. If you are not sure why your spasticity has changed, speak to your health providers for further testing.
Spasticity is related to several changes to the body that happen after SCI. The main reason for spasticity after SCI is a reduced ability of the brain to ‘calm down’ overactive reflexes. Over time, the muscles and tendons may also change, becoming more tense and stiff, which also contributes to the symptoms of spasticity.
The stretch reflex
The stretch reflex is an automatic movement response that happens when a muscle is stretched quickly, causing the muscle to tense. It is commonly tested as the ‘tendon tap’ below the kneecap.
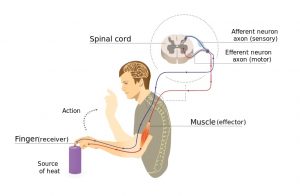
Pain signals from touching something hot travels to the spinal cord and back to the muscles without going to the brain first.4
When a muscle is quickly stretched, it activates special stretch sensors called muscle spindles. They send a signal through sensory neurons to the spinal cord. In the spinal cord, the message is passed along to motor neurons, which send a movement command back to the muscle, causing it to contract. This reflex happens in the spinal cord without travelling to the brain first.
Like muscle stretch, pain can also trigger spinal cord reflexes that use the same nerve pathway as the stretch reflex. For example, stepping on something sharp or touching a hot burner activates spinal cord reflexes.
The brain normally dampens spinal cord reflexes
Although the stretch reflex happens in the spinal cord, the brain influences how sensitive the reflex is. The brain normally sends signals down the spinal cord, which dampens the sensitivity of reflexes.
This is called descending inhibition. ‘Descending’ means ‘coming down from the brain’, and ‘inhibition’ means ‘reducing the activity of’ the stretch reflex. Descending inhibition is important because it tells the stretch reflex to ‘calm down’ so it doesn’t get in the way of normal movements.
Spinal cord injury prevents the brain from dampening spinal cord reflexes
When the spinal cord is injured, descending inhibition from the brain is cut off. Without its calming effects,
the stretch reflex becomes overactive. This can lead to a constant level of muscle tension (called muscle tone) and excessive reflexes which cause the muscles to tighten uncontrollably or unexpectedly and the other symptoms of spasticity.
Watch SCIRE’s YouTube video explaining why spasticity happens after SCI.5
The main way that spasticity is diagnosed and monitored is through a physical examination. Your health providers will talk to you about your symptoms, functional abilities, and current treatment plan, look at your muscles and posture, and test the muscles in various ways. This may include:
- Hands-on tests where the joints are moved slowly and quickly
- Active tests of strength and movement
- Testing your reflexes
Your health providers may also observe tasks like walking, transferring, and eating – this can help them understand how spasticity affects you in your everyday life.
Health providers often use special collections of questions and tests called outcome measures, which help them accurately keep track of changes in spasticity. Spasticity may change over time so regular check-ins with your health team, especially while figuring out what works best for you, are often an important part of managing your spasticity.
Spasticity can negatively affect the health and wellness of some people, but it can sometimes have benefits as well. It is important to determine whether your spasticity is a problem for you. Treating spasticity unnecessarily can have drawbacks, such as unwanted side effects, costs, and time. It is important that you discuss your treatment options and weigh the pros and cons of treating spasticity together with your health team to determine the best course of action for you.
Problems with spasticity
 Muscle spasms and reflexes can contribute to a number of potential problems, such as:
Muscle spasms and reflexes can contribute to a number of potential problems, such as:
- Pain
- Sleep problems
- Reduced mobility and function
- Difficulties maintaining posture and positioning
- Skin breakdown and hygiene concerns
- Bladder and bowel accidents
- Joint contractures
- Sexual and reproductive health issues
- Difficulties with care
Benefits of spasticity
Spasticity can also have some benefits for people with SCI, which may include:
- Better mobility, standing, and walking
- Assistance with transfers (such as supporting the body weight while transferring from a wheelchair to a bed or chair)
- Preventing muscle wasting or weakening due to inactivity
- Improved circulation
- Intentionally triggered spasms can help to empty the bowel and bladder in people with certain types of bowel or bladder problems
- Reflex erections during sexual activity
- It may serve as a warning sign of infections or other health issues
YouTube video about the downfalls of treating non-problematic spasticity.8
There are many different treatments for spasticity. Every person’s spasticity is different, so finding the best treatment or combination of treatments often involves trial and error.
Spasticity treatment usually starts with conservative treatments such as positioning and maintaining good muscle length. If these do not provide enough relief, spasticity medications and injections may be recommended. Surgical treatments are considered as a last option for severe spasticity.
Avoiding spasticity triggers
An important part of managing spasticity is learning how to manage your spasticity triggers. Spasticity is often triggered by bladder, bowel, skin, or other health issues, so maintaining good overall health and taking care of these issues is an important part of managing spasticity. Speak to your health providers about optimizing your self-care routines to prevent spasticity.
Movement and therapeutic treatment options
There are a number of different movement, hands-on, and electrical treatments that may be done on your own, with a caregiver, or in conjunction with a therapist. These treatments generally produce fewer side effects than medications or surgeries; however, they also tend to have short-term effects.
Posture and positioning
Good posture and positioning may help to keep the muscles at an appropriate length and help prevent contractures. You may need to work with your health providers to determine the best positions and equipment to manage your spasticity.
Stretching and range of motion
Stretching and range of motion exercises are commonly used treatments to reduce spasticity and minimize complications like contractures after SCI. Stretching is often achieved through prolonged positioning, such as placing a wedge between the knees to stretch the hips.
Bracing and casting
 Various braces, orthoses, and casts may be used to maintain proper positioning of the arms and legs to help reduce spasticity, improve function, and prevent complications.
Various braces, orthoses, and casts may be used to maintain proper positioning of the arms and legs to help reduce spasticity, improve function, and prevent complications.
Standing
Standing can provide a prolonged stretch to certain muscles, such as the calf and hamstring muscles, which may help with spasticity. For some people, standing may be done using specialized equipment such as tilt tables, standing frames, and standing wheelchairs.
Neurodevelopmental therapy (NDT)
Neurodevelopmental therapy (NDT, sometimes called Bobath therapy) is a type of physiotherapy and occupational therapy treatment where a therapist uses hands-on techniques to guide a person through movements. It is used to help practice quality functional movements.
Walking
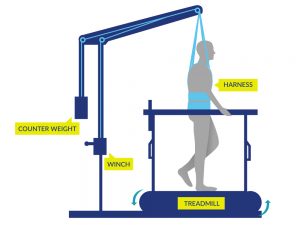 Walking may be done by some people (typically with incomplete SCI) with or without gait aids or assistance from health providers or with the use of specialized equipment such as body weight supported treadmill training or robotic exoskeletons.
Walking may be done by some people (typically with incomplete SCI) with or without gait aids or assistance from health providers or with the use of specialized equipment such as body weight supported treadmill training or robotic exoskeletons.
Functional electrical stimulation (FES) exercise
Functional electrical stimulation (FES) involves the use of electrical stimulation to activate specific muscles of the arms or legs during an activity such as stationary cycling, arm exercises or walking.
See our article on FES for more information!
Massage
Massaging muscles may help to stimulate the sensory nerves, which are part of the reflex spasticity response.
Transcutaneous electrical nerve stimulation (TENS)
Transcutaneous electrical nerve stimulation (TENS) involves the use of electrodes placed on the skin to stimulate the sensory nerves without producing muscle tension.
See our article on TENS for more information!
Do movement and therapeutic treatment options work?
Although many of these treatments are commonly used in the treatment of spasticity, the research is unclear about whether a number of these therapies, including stretching and range of motion, standing, neurodevelopmental therapy, and massage; are actually effective for reducing spasticity after SCI. However, many of these treatments often have several therapeutic purposes after SCI (such as reducing pain or preventing contractures), which may explain their widespread use. Further research is needed to better understand the effects of these treatments on spasticity.
However, there is evidence that body weight supported treadmill training, robotic exoskeleton walking, functional electrical stimulation (FES) exercise, and TENS are effective treatments for reducing spasticity after SCI.
Medications
Oral medications are typically prescribed for the treatment of widespread spasticity. Finding the right medication may involve trial and error and involves working closely with your doctor to find the best fit for you.
Baclofen (tablets and baclofen pumps)
Baclofen (Lioresal) is a muscle relaxant that is commonly used to treat spasticity. It can be taken as tablets by mouth or administered into the sac surrounding the spinal cord (called intrathecal baclofen) through a surgically implanted baclofen pump. Baclofen is effective for treating spasticity after SCI. However, it can have several side effects such as dizziness, drowsiness, anxiety, confusion, and weakness. Extra care is also needed when discontinuing therapy to avoid withdrawal symptoms. Baclofen is the most common medication prescribed for spasticity after SCI.
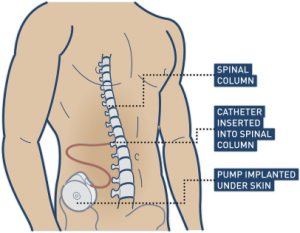
Baclofen is commonly used for controlling spasticity after a spinal cord injury. This can be achieved by surgically implementing a pump that connects directly to the spinal cord.11
Watch “Intrathecal Baclofen for Reducing Spasticity After Spinal Cord Injury” from our SCIRE video series on Neuromodulation!
Other spasticity medications
A number of other medications are used clinically or have been studied for their effects on spasticity after SCI. Speak to your doctor or pharmacist for more information about these medications.
Medications that are effective for spasticity after SCI:
Medications that may be effective for spasticity after SCI:
- Cannabinoid medications (Dronabinol and Nabilone)
- Gabapentin
- Orphenadrine Citrate
- Diazepam
- Dantrolene
Medications that are not supported for treating spasticity after SCI:
- Fampridine (4-Aminopyridine)
- Levetiracetam
Injections
Injections into the nerves and muscles may be used to help manage localized areas of spasticity.
Botulinum toxin (Botox) injections
 Botulinum toxin is a toxin that can cause muscle paralysis. Very small doses of certain strains of botulinum toxin can be injected into muscles to treat spasticity. It is commonly known for its cosmetic use by its trade names Botox, Dysport, and Xeomin. Botulinum toxin injections are temporary, with effects that wear off over time (usually around 3 to 6 months). Research evidence supports that botulinum toxin is effective in reducing focal spasticity after SCI.
Botulinum toxin is a toxin that can cause muscle paralysis. Very small doses of certain strains of botulinum toxin can be injected into muscles to treat spasticity. It is commonly known for its cosmetic use by its trade names Botox, Dysport, and Xeomin. Botulinum toxin injections are temporary, with effects that wear off over time (usually around 3 to 6 months). Research evidence supports that botulinum toxin is effective in reducing focal spasticity after SCI.
See our article on Botulinum Toxin for more information
Phenol injections
Phenol injections involve injecting a type of alcohol into nerves which supply the spastic muscle. Phenol damages the nerve axons, so the nerves cannot send signals to the muscles that cause spasticity. This procedure is also sometimes used with another alcohol, ethanol. Phenol injections may be effective for reducing spasticity after SCI.
Surgical treatments
 Surgery is typically reserved for and joint contractures are impacting care, function and quality of life
Surgery is typically reserved for and joint contractures are impacting care, function and quality of life
Tendon releases or transfers
Tendon releases are surgeries that lengthen shortened tendons (the part of the muscle that attaches to a bone) affected by spasticity. Tendon transfers involve surgically moving tendons that attach to muscles. These techniques can assist with better positioning of the feet or arms when excessive spasticity interferes with safe or appropriate positioning. However, there is limited research investigating the specific effects with SCI.
Myelotomy and Rhizotomy
Myelotomy and rhizotomy are surgical procedures that involve intentionally damaging part of the spinal cord (myelotomy) or nerve (rhizotomy) to reduce spasticity. Damaging the nerve fibers related to spasticity can prevent them from communicating and causing unwanted muscle spasms. These techniques are not common because they are permanent and invasive. They are only used for severe and intolerable spasticity that does not respond to other treatments. Myelotomy is effective for reducing spasticity after SCI.
Other treatments
A number of other medical, alternative, and self-management treatments may be used to manage spasticity. There is some limited evidence that other treatments, such as transcranial magnetic stimulation (TMS), hippotherapy (therapeutic horseback riding), and others may help to treat spasticity after SCI. However, these treatments are not typically used or available in standard practice at this time. Speak with your health providers about any treatments you are considering trying as a treatment for your spasticity.
The research evidence suggests that conservative treatments that involve active movement and electrical stimulation help to reduce spasticity short-term after SCI. It is not clear whether passive movement therapies like stretching help treat spasticity.
Medications and injections may include baclofen and botulinum toxin injections, which are effective for treating spasticity but may have additional side effects. There are many other drugs and treatments that may require further research. Surgery may be considered as a last resort if other treatments fail.
It is important to discuss any questions of concerns that you have about your treatment options in detail with your health providers to find the best management options for you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Professional “Spasticity” Module:
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Curt A, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0.
Available from: https://scireproject.com/evidence/spasticity/
Evidence for “Movement and physical therapies” is based on the following studies:
Stretching and range of motion
[1] Skold C. Spasticity in spinal cord injury: self-and clinically rated intrinsic fluctuations and intervention-induced changes. Arch Phys Med Rehabil 2000;81:144-9.
[2] Kakebeeke T, Lechner H, Knapp P. The effect of passive cycling movements on spasticity after spinal cord injury: preliminary results. Spinal Cord 2005;43:483-8.
Standing
[1] Odeen I & Knutsson E. Evaluation of the effects of muscle stretch and weight load in patients with spastic paraplegia. Scand J Rehabil Med 1981;13:117-21.
[2] Short-term effects of surface electrical stimulation. Arch Phys Med Rehabil 1988b;69:598-604.
[3] Adams M, Hicks A. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med 2011;34:488-94.
Neurodevelopmental Therapy
[1] Li S, Xue S, Li Z, Liu X. Effect of baclofen combined with neural facilitation technique on the reduction of muscular spasm in patients with spinal cord injury. Neur Regen Res 2007;2:510-2.
Walking
[1] Fang C, Hsu M, Chen C, Cheng H, Chou C, Chang Y. Robot-assisted passive exercise for ankle hypertonia in individuals with chronic spinal cord injury. J Med Biol Eng 2015;35:464-72.
[2] Mirbagheri M, Kindig M, Niu X. Effects of robotic-locomotor training on stretch reflex function and muscular properties in individuals with spinal cord injury. Clinical Neurophysiol 2015;126:997-1006.
[3] Manella K & Field-Fote E. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci 2013;31:633-46.
Functional Electrical Stimulation
[1] Kapadia N, Masani K, Craven B, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med 2014;37:511-24.
[2] Manella K & Field-Fote E. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci 2013;31:633-46.
[3] Ralston K, Harvey L, Batty J, et al. Functional electrical stimulation cycling has no clear effect on urine output, lower limb swelling, and spasticity in people with spinal cord injury: A randomised cross-over trial. J Physiother 2013;59:237-43.
[4] Kuhn D, Leichtfried V, Schobersberger W. Four weeks of functional electrical stimulated cycling after spinal cord injury: a clinical cohort study. Inter J Rehabil Res 2014;37:243-50.
[5] Sadowsky C, Hammond E, Strohl A, et al. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med 2013;36:623-31.
[6] Reichenfelser W, Hackl H, Hufgard J, Kastner J, Gstaltner K, Gföhler M. Monitoring of spasticity and functional ability in individuals with incomplete spinal cord injury with a functional electrical stimulation cycling system. J Rehabil Med 2012;44:444-9.
[7] Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil 2008;22:627-34
[8] Mirbagheri M, Ladouceur M, Barbeau H, Kearney R. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. Trans Neural Syst Rehabil Eng 2002;10:280-9.
[9] Granat M, Ferguson A, Andrews B, Delargy M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury–observed benefits during gait studies. Paraplegia 1993;31:207-15.
[10] Thoumie P, Le C, Beillot J, Dassonville J, Chevalier T, Perrouin-Verbe B et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia 1995;33:654-9.
Transcutaneous Electrical Nerve Stimulation
[1] Oo W. Efficacy of addition of transcutaneous electrical nerve stimulation to standardized physical therapy in subacute spinal spasticity: a randomized controlled trial. Arch Phys Med Rehabil 2014;95:2013-20.
[2] Chung B & Cheng, B. Immediate effect of transcutaneous electrical nerve stimulation on spasticity in patients with spinal cord injury. Clinical Rehabilitation 2010;24:202-210.
[3] Aydin G, Tomruk S, Keleş I, Demir SO, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil. 2005 Aug;84(8):584-92.
Massage
[1] Goldberg J, Seaborne D, Sullivan S, Leduc B. The effect of therapeutic massage on H-reflex amplitude in persons with a spinal cord injury. Phys Ther 1994;74:728-37.
Evidence for “Medications” is based on the following studies:
Baclofen
[1] Chu V, Hornby T, Schmit B. Effect of antispastic drugs on motor reflexes and voluntary muscle contraction in incomplete spinal cord injury. Arch Phys Med Rehabil 2014;95:622-32.
[2] Nance P, Huff F, Martinez-Arizala A, Ayyoub Z, Chen D, Bian A, Stamler D. Efficacy and safety study of arbaclofen placarbil in patients with spasticity due to spinal cord injury. Spinal Cord 2011;49:974-80.
[3] Aydin G, Tomruk S, Keles I, Demir S, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil 2005;84:584-92.
[4] Duncan G, Shahani B, Young R. An evaluation of baclofen treatment for certain symptoms in patients with spinal cord lesions. A double-blind, cross-over study. Neurology 1976;26:441-6.
[5] Burke D, Gillies J, Lance J. An objective assessment of a gamma aminobutyric acid derivative in the control of spasticity. Proc Aust Assoc Neurol 1971;8:131-4.
[6] Dicpinigaitis P, Allusson V, Baldanti A, and Nalamati J. Ethnic and gender differences in cough reflex sensitivity. Respiration 2001;68:480-2.
[7] Veerakumar A, Cheng J, Sunshine A, Ye X, Zorowitz R, Anderson W. Baclofen dosage after traumatic spinal cord injury: a multi-decade retrospective analysis. Clin Neurol Neurosurg 2015;129:50-6.
[8] Nance P. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc 1994;17:150-6.
Intrathecal Baclofen
[1] Ordia J, Fischer E, Adamski E, Spatz E. Chronic intrathecal delivery of baclofen by a programmable pump for the treatment of severe spasticity. J Neurosurg 1996;85:452-7.
[2] Nance P, Schryvers O, Schmidt B, Dubo H, Loveridge B, Fewer D. Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci 1995;22:22-9.
[3] Coffey J, Cahill D, Steers W, Park T, Ordia J, Meythaler J, et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg 1993;78:226-32.
[4] Hugenholtz H, Nelson R, Dehoux E, Bickerton R. Intrathecal baclofen for intractable spinal spasticity-a double-blind cross-over comparison with placebo in 6 patients. Can J Neurol Sci 1992;19:188-95.
[5] Loubser P, Narayan R, Sandin K, Donovan W, Russell K. Continuous infusion of intrathecal baclofen: long-term effects on spasticity in spinal cord injury. Paraplegia 1991;29:48-64.
[6] Penn R, Savoy S, Corcos D, Latash M, Gottlieb G, Parke B et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989;320:1517-21.
[7] Boviatsis E, Kouyialis A, Korfias S, Sakas D. Functional outcome of intrathecal baclofen administration for severe spasticity. Clin Neurol Neurosurg 2005;107:289-95.
[8] Azouvi P, Mane M, Thiebaut J, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil 1996;77:35-9.
[9] Plassat R, Perrouin Verbe B, Menei P, Menegalli D, Mathe J, Richard I. Treatment of spasticity with intrathecal baclofen administration: Long-term follow-up review of 40 patients. Spinal Cord 2004;42:686-93.
[10] Zahavi A, Geertzen J, Middel B, Staal M, Rietman J. Long term effect (more than five years) of intrathecal baclofen on impairment, disability, and quality of life in patients with severe spasticity of spinal origin. J Neurol Neurosurg Psychi 2004;75:1553-7.
[11] Korenkov A, Niendorf W, Darwish N, Glaeser E, Gaab M. Continuous intrathecal infusion of baclofen in patients with spasticity caused by spinal cord injuries. Neurosurg Rev 2002;25:228-30.
[12] Broseta J, Garcia-March G, Sanchez-Ledesma M, Anaya J, Silva I. Chronic intrathecal baclofen administration in severe spasticity. Stereotact Funct Neurosurg 1990;54-55:147-53.
[13] Parke B, Penn R, Savoy S, Corcos D. Functional outcome after delivery of intrathecal baclofen. Arch Phys Med Rehabil 1989;70:30-2.
Tizanidine
[1] Chu et al. 2014.
[2] Nance P, Bugaresti J, Shellenberger K, Sheremata W, Martinez-Arizala A. Efficacy and safety of tizanidine in the treatment of spasticity in patients with spinal cord injury. Neurol 1994;44:S44-51.
[3] Mirbagheri M, Kindig M, Niu X, Varoqui D. Therapeutic effects of anti-spastic medication on neuromuscular abnormalities in SCI: A system identification approach. IEEE EMBS 2013;6203-6.
Clonidine
[1] Stewart J, Barbeau H, Gauthier S. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Can J Neurol Sci 1991;18:321-32.
[2] Malinovsky J, Malinge M, Lepage J, Pinaud M. Sedation caused by clonidine in patients with spinal cord injury. Bri J Anaesthesia 2003;90:742-5.
[3] Remy-Neris O, Barbeau H, Daniel O, Boiteau F, Bussel B. Effects of intrathecal clonidine injection on spinal reflexes and human locomotion in incomplete paraplegic subjects. Exp Brain Res 1999;129:433-40.
4-Aminopyridine
[1] Cardenas D, Ditunno J, Graziani V, Jackson A, Lammertse D, Potter P, et al. Phase 2 trial of sustained-release fampridine in chronic spinal cord injury. Spinal Cord 2007;45:158-68.
[2] Cardenas D, Ditunno JF, Graziani V, et al. Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal Cord 2014;52:70-76.
[3] Potter P, Hayes K, Segal J, Hsieh J, Brunnemann S, Delaney G, et al. Randomized double-blind crossover trial of fampridine-SR (sustained release 4-aminopyridine) in patients with incomplete spinal cord injury. J Neurotrauma 1998a;15:837-49.
[4] Potter P, Hayes K, Hsieh J, Delaney G, Segal J. Sustained improvements in neurological function in spinal cord injured patients treated with oral 4-aminopyridine: Three cases. Spinal Cord 1998b;36:147-55.
[5] Donovan W, Halter J, Graves D, Blight A, Calvillo O, McCann M, et al. Intravenous infusion of 4-AP in chronic spinal cord injured subjects. Spinal Cord 2000;38:7-15.
[6] Hayes K, Potter P, Wolfe D, Hsieh J, Delaney G, Blight AR. 4-Aminopyridine-sensitive neurologic deficits in patients with spinal cord injury. J Neurotrauma 1994;11:433-46.
Cyproheptadine
[1] Thompson C, Hornby T. Divergent modulation of clinical measures of volitional and reflexive motor behaviors following serotonergic medications in human incomplete spinal cord injury. J Neurotrauma. 2013;30:498-502.
[2] Nance et al. 1994.
[3] Meythaler J, Roper J, Brunner R. Cyproheptadine for intrathecal baclofen withdrawal. Arch Phys Med Rehabil 2003;84:638-42.
Gabapentin
[1] Gruenthal M, Mueller M, Olson W, Priebe M, Sherwood A, Olson W. Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord 1997;35:686-9.
Orphenadrine Citrate
[1] Casale R, Glynn C, Buonocore M. Reduction of spastic hypertonia in patients with spinal cord injury: a double-blind comparison of intravenous orphenadrine citrate and placebo. Arch Phys Med Rehabil 1995;76:660-5.
Cannabinoids
[1] Pooyania S, Ethans K, Szturm T, Casey A, Perry D. A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury. Arch Phys Med Rehabil 2010;91:703-7.
[2] Hagenbach U, Luz S, Ghafoor N, Berger J, Grotenhermen F, Brenneisen R, et al. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord 2007;45:551-62.
[3] Kogel R, Johnson P, Chintam R, Robinson C, Nemchausky B. Treatment of spasticity in spinal cord injury with dronabinol, a tetrahydrocannabinol derivative. Am J Ther 1995;2:799-805.
Evidence for “Injections” is based on the following studies:
[1] Richardson D, Sheean G, Werring D, Desai M, Edwards S, Greenwood R et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychi 2000;69:499-506.
[2] Spiegl U, Maier D, Gonschorek O, Heyde C, Buhren V. Antispastic therapy with botulinum toxin type A in patients with traumatic spinal cord lesion. GMS Interdiscip Plast Reconstr Surg 2014;3:1-5.
[3] Bernuz B, Genet F, Terrat P, et al. Botulinum toxin effect on voluntary and stretch reflex-related torque produced by the quadriceps: An isokinetic pilot study. Neurorehabil Neural Repair 2012;26:542-7.
[4] Hecht M, Stolze H, uf dem B, Giess R, Treig T, Winterholler M et al. Botulinum neurotoxin type A injections reduce spasticity in mild to moderate hereditary spastic paraplegia–report of 19 cases. Mov Disord 2008;23:228-33.
[5] Uchikawa K, Toikawa H, Liu M. Subscapularis motor point block for spastic shoulders in patients with cervical cord injury. Spinal Cord 2009;47:249-51.
[6] Ghai A, Sangwan S, Hooda S, Garg N, Kundu Z, Gupta T. Evaluation of interadductor approach in neurolytic blockade of obturator nerve in spastic patients. Saudi J Anaesth 2013;7:420-6.
[7] Ghai A, Sangwan S, Hooda S, Kiran S, Garg N. Obturator neurolysis using 65% alcohol for adductor muscle spasticity. Saudi J Anaesth 2012;6:282-4.
[8] Yasar E, Tok F, Taskaynatan M, Yilmaz B, Balaban B, Alaca R. The effects of phenol neurolysis of the obturator nerve on the distribution of buttock-seat interface pressure in spinal cord injury patients with hip adductor spasticity. Spinal Cord 2010;48:828-31.
Evidence for “Surgeries” is based on the following studies:
[1] Livshits A, Rappaport Z, Livshits V, Gepstein R. Surgical treatment of painful spasticity after spinal cord injury. Spinal Cord 2002;40:161-6.
[2] Putty T & Shapiro S. Efficacy of dorsal longitudinal myelotomy in treating spinal spasticity: a review of 20 cases. J Neurosurg 1991;75:397-401.
Other references:
Abel NA, Smith RA. Intrathecal baclofen for treatment of intractable spinal spasticity. Arch Phys Med Rehabil 1994; 75:54-58.
Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord 2005;43(10):577-586.
Al-Khodairy AT, Gobelet C, Rossier AB. Has botulinum toxin type A a place in the treatment of spasticity in spinal cord injury patients? Spinal Cord 1998; 36(12):854-858.
Avellino AM, Loeser JD. Intrathecal baclofen for the treatment of intractable spasticity of spine or brain etiology. Neuromodulation 2000; 3(2):75-81.
Bajd T, Gregoric M, Vodovnik L, Benko H. Electrical stimulation in treating spasticity resulting from spinal cord injury. Arch Phys Med Rehabil 1985; 66(8):515-517.
Bakheit AM, Pittock S, Moore AP, Wurker M, Otto S, Erbguth F et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. Eur J Neurol 2001; 8(6):559-565.
Bohannon RW. Tilt table standing for reducing spasticity after spinal cord injury. Arch Phys Med Rehabil 1993; 74(10):1121-1122.
Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine 2001; 26(24 Suppl):S146-S160.
Canadian Paraplegic Association. Workplace participation national survey of Canadians with SCI. Canadian Paraplegic Association http://www.canparaplegic.org/en/Employment_and_Education_24/EMPLOYMENT_6/14.html. 1996; Last accessed: 9-15-2008.
Corry IS, Cosgrove AP, Walsh EG, McClean D, Graham HK. Botulinum toxin A in the hemiplegic upper limb: a double-blind trial. Dev Med Child Neurol 1997; 39(3):185-193.
Elbasiouny, S. M., Moroz, D., Bakr, M. M., & Mushahwar, V. K. (2010). Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabilitation and neural repair, 24(1), 23-33.
Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord 2008; 46(2):96-102.
Goulet C, Arsenault AB, Bourbonnais D, Laramee MT, Lepage Y. Effects of transcutaneous electrical nerve stimulation on H-reflex and spinal spasticity. Scand J Rehabil Med 1996; 28(3):169-176.
Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl 1997; 6:S92-120.
Granat MH, Ferguson AC, Andrews BJ, Delargy M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury–observed benefits during gait studies. Paraplegia 1993; 31(4):207-215.
Gruenthal M, Mueller M, Olson WL, Priebe MM, Sherwood AM, Olson WH. Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord 1997; 35(10):686-689.
Hayes KC. Fampridine-SR for multiple sclerosis and spinal cord injury. Expert Rev Neurother 2007; 7(5):453-461.
Heetla HW, Staal MJ, Kliphuis C, van Laar T. The incidence and management of tolerance in intrathecal baclofen therapy. Spinal Cord, 2009; 47:751-756.
Heetla HW, Staal MJ, Kliphuis C, van Laar T. Tolerance to continuous intrathecal baclofen infusion can be reversed by pulsatile bolus infusion. Spinal Cord, 2010; 48: 483-486.
Hidler, J. M., & Rymer, W. Z. (1999). A simulation study of reflex instability in spasticity: origins of clonus. IEEE Transactions on Rehabilitation Engineering, 7(3), 327-340.
Hinderer SR, Lehmann JF, Price R, White O, deLateur BJ, Deitz J. Spasticity in spinal cord injured persons: quantitative effects of baclofen and placebo treatments. Am J Phys Med Rehabil 1990; 69(6):311-317.
Hinderer SR. The supraspinal anxiolytic effect of baclofen for spasticity reduction. Am J Phys Med Rehabil 1990; 69(5):254-258.
Hyman N, Barnes M, Bhakta B, Cozens A, Bakheit M, Kreczy-Kleedorfer B et al. Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry 2000; 68(6):707-712.
Kesiktas N, Paker N, Erdogan N, Gulsen G, Bicki D, Yilmaz H. The use of hydrotherapy for the management of spasticity. Neurorehabil Neural Repair 2004; 18(4):268-273.
Kirshblum S. Treatment alternatives for spinal cord injury related spasticity. J Spinal Cord Med 1999; 22(3):199-217.
Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD et al. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med 2005; 28(3):241-245.
Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clinical Rehabilitation 2008; 22(7):627-634.
Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993; 74:73-78.
Lechner HE, Feldhaus S, Gudmundsen L, Hegemann D, Michel D, Zach GA et al. The short-term effect of hippotherapy on spasticity in patients with spinal cord injury. Spinal Cord 2003; 41(9):502-505.
Levi R, Hultling C, Nash MS, Seiger A. The Stockholm spinal cord injury study: 1. medical problems in a regional SCI population. Paraplegia 1995; 33:308-315.
Maurer M, Henn V, Dittrich A, Hofmann A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. Eur Arch Psychiatry Clin Neurosci 1990; 240(1):1-4.
Maynard FM, Karunas RS, Waring WP, III. Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 1990; 71(8):566-569.
Midha M, Schmitt JK. Epidural spinal cord stimulation for the control of spasticity in spinal cord injury patients lacks long-term efficacy and is not cost-effective. Spinal Cord 1998; 36(3):190-192.
Mirbagheri MM, Ladouceur M, Barbeau H, Kearney RE. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. IEEE Trans Neural Syst Rehabil Eng 2002; 10(4):280-289.
Nance PW, Shears AH, Nance DM. Reflex changes induced by clonidine in spinal cord injured patients. Paraplegia 1989; 27(4):296-301.
Ochs G, Struppler A, Meyerson BA, Linderoth B, Gybels J, Gardner BP et al. Intrathecal baclofen for long-term treatment of spasticity: a multi-centre study. J Neurol Neurosurg Psychiatry 1989; 52:933-939.
Penn RD. Intrathecal baclofen for spasticity of spinal origin: seven years of experience. J Neurosurg 1992 ;77:236-240.
Possover M, Schurch B, Henle KP. New strategies of pelvic nerves stimulation for recovery of pelvic visceral functions and locomotion in paraplegics. Neurourol Urodyn 2010 Nov; 29(8).
Rayegani, S. M., Shojaee, H., Sedighipour, L., Soroush, M. R., Baghbani, M., & Amirani, O. B. (2011). The effect of electrical passive cycling on spasticity in war veterans with spinal cord injury. Frontiers in Neurology; 2(39):1-7.
Richardson D, Edwards S, Sheean GL, Greenwood RJ, Thompson AJ. The effect of botulinum toxin on hand function after incomplete spinal cord injury at the level of C5/6: a case report. Clin Rehabil 1997; 11(4):288-292.
Shields RK, Dudley-Javoroski S. Monitoring standing wheelchair use after spinal cord injury: a case report. Disabil Rehabil 2005; 27:142-146.
Simpson DM, Alexander DN, O’Brien CF, Tagliati M, Aswad AS, Leon JM et al. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology 1996; 46(5):1306-1310.
Simpson DM. Clinical trials of botulinum toxin in the treatment of spasticity. Muscle Nerve Suppl 1997; 6:S169-S175.
Smith SJ, Ellis E, White S, Moore AP. A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin Rehabil 2000; 14(1):5-13.
Snow BJ, Tsui JK, Bhatt MH, Varelas M, Hashimoto SA, Calne DB. Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol 1990; 28(4):512-515.
Thoumie P, Le CG, Beillot J, Dassonville J, Chevalier T, Perrouin-Verbe B et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia 1995; 33(11):654-659.
Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatry 1990; 53(9):754-763.
Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R et al. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev 2002; 39:53-61.
Wasiak J, Hoare B, Wallen M. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy. Cochrane Database Syst Rev 2004; (4):CD003469.
Lechner H, Kakebeeke T, Hegemann D, Baumberger M. The effect of hippotherapy on spasticity and on mental well-being of persons with spinal cord injury. Arch Phys Med Rehabil 2007;88:1241-8.
Nardone R, Holler Y, Thomschewski A, et al. rTMS modulates reciprocal inhibition in patients with traumatic spinal cord injury. Spinal Cord 2014;52:831-5.
Benito J, Kumru H, Murillo N, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spin Cord Injury Rehabil 2012;18:106-12.
Kumru H, Vidal J, Kofler M, Portell E, Valls-Sole J. Alterations in excitatory and inhibitory brainstem interneuronal circuits after severe spinal cord injury. Journal of Neurotrauma, 2010;27:721-8.
Image credits:
- Muscle strain ©Kylie Mhai, CC BY 3.0 US
- Modified from: Dorsiplantar ©Connexions, CC BY 3.0
- Image by SCIRE Community Team
- Imgnotraçat arc reflex eng ©MartaAguayo, CC BY-SA 3.0
- Image by SCIRE Community Team
- Image by SCIRE Community Team
- Insomnia ©Gan Khoon Lay, CC BY 3.0 US
- Image by SCIRE Community Team
- Standing frame ©Memasa, CC BY-SA 3.0
- Image by SCIRE Community Team
- Intrathecal-pump-cartoon ©Anand Swaminathan, CC BY-SA 3.0
- Pills ©Nikita Kozin, CC BY 3.0 US
- Treatment ©Royal@design, CC BY 3.0 US
- Surgery ©Healthcare Symbols, CC0 1.0
- Neuro-ms ©Baburov, CC BY-SA 4.0