Author: SCIRE Community Team | Reviewer: Patricia Mills | Published: 21 November 2017 | Updated: ~
Baclofen is a medication that is used to treat spasticity. This page provides basic information about baclofen and its use after spinal cord injury (SCI).
Key Points
- Baclofen (Lioresal) is a medication that is used to relax muscles affected by spasticity.
- Baclofen is derived from gamma aminobutyric acid (GABA), a chemical in the body that helps to reduce reflexes that are responsible for spasticity.
- Baclofen can be taken by mouth as a tablet or injected into the spinal canal in a liquid form through an implanted pump (an intrathecal baclofen pump).
- Research evidence supports that both baclofen tablets and baclofen pumps are effective to reduce spasticity after SCI.
Baclofen is a medication that is used to treat spasticity. It is also known by the trade name Lioresal. Baclofen is a muscle relaxant medication that helps to reduce muscle tension and spasms caused by nervous system disorders like spinal cord injury and multiple sclerosis.
Baclofen is derived from a chemical called gamma aminobutyric acid (GABA), which reduces muscle activity. It can enter into the brain and spinal cord, where it helps to reduce reflexes responsible for spasticity. Baclofen can be taken by mouth as a tablet or injected into the spinal canal as a fluid using an implanted baclofen pump.
Baclofen in tablet form is usually the first type of medication used to treat spasticity after SCI. There is strong evidence that oral baclofen improves the symptoms of spasticity
Baclofen administered by intrathecal pump is usually a last option that is explored because of the surgery that is required to implant the pump. However, when it is used, there is strong evidence that intrathecal baclofen is effective to treat the symptoms of spasticity in people with SCI.
Baclofen is a prescription medication that is given with specific instructions from your health providers on how to take it. It is important to follow their instructions closely when taking this medication and discuss any questions you have about your use of the medication directly with your team.
Baclofen tablets
Baclofen is usually taken by mouth as a tablet. Baclofen is prescribed at a unique dose for you and then carefully monitored. Treatment is usually started with a trial of a low dose of the drug to find out if it works and then slowly increased to determine the optimal dose. This dose will then be maintained while continuing to take the drug.
You can expect some side effects when starting Baclofen (and any other anti-spasticity drug), so do not be surprised if that should happen. As your body gets used to the new drug, the side effects can improve and in some cases completely resolve. Side effects, if they occur, usually are experienced before the drug starts to work on the spasticity therefore it is important to stay on the drug as long as the side effects are tolerable. If the side effects have not improved or are not tolerable by the end of 2 weeks of starting the new drug, and you don’t feel that the benefit of the drug is worth the side effects that you are experiencing, then notify your physician as you will likely have to either decrease the dose or consider trying another drug instead.
Baclofen pumps (Intrathecal baclofen)
Baclofen may also be injected directly into the sac that surrounds the spinal cord. This is called intrathecal baclofen. ‘Intrathecal’ means ‘within the spinal sac’ (also called the thecal sac).
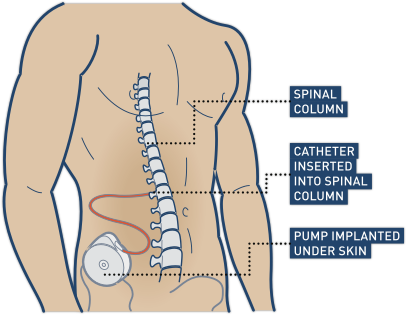
Intrathecal baclofen is usually administered using a surgically implanted pump that is placed under the skin near the abdomen called a baclofen pump. The pump is then connected to the spinal cord fluid through a thin tube (catheter) that travels through your soft tissue underneath the skin. The pump provides a dose of the medication through the catheter at regular intervals according to its settings.
Baclofen pumps are first managed by a health provider in a hospital setting in the early days following surgical implantation. Then, the device can be programmed to release a programmed dose of baclofen throughout the day for use at home.
Regular visits to the intrathecal baclofen pump doctor are required to refill the pump and monitor for any problems. Therefore, in order for you to be a candidate for getting the pump, you need to be able to travel from where you live to where the pump can be serviced. The pump can be removed if you decide you no longer would like to receive the treatment.
Increasing oral baclofen dosage may result in a number of side effects like sleepiness. The solution is a pump implanted under the skin that administers baclofen directly to the spinal cord, aka “intrathecally”.
When are baclofen pumps used?
Typically, intrathecal baclofen is recommended when spasticity is severe and widespread throughout the body, and other approaches to manage your spasticity, such as medications by mouth, have not worked. Much lower doses of baclofen are used when given as an intrathecal injection. This may help people with severe spasticity to manage spasticity more effectively, and usually results in no side effects.
However, it is important to know that complications with the pump can occur, potentially causing episodes of too much baclofen (baclofen overdose) or too little baclofen (baclofen withdrawal) to be delivered. Therefore, it is important to consult with an intrathecal baclofen pump expert in order to determine if the pump is a good option for you.
Baclofen is not appropriate for everyone. There are certain situations in which it may not be safe to use. This is not a complete list; please consult a health provider for detailed safety information before using this treatment.
Baclofen should not be used in the following situations:
- By people with health conditions such as epilepsy, kidney problems, diabetes, or breathing problems
- By people with conditions that cause confusion or depression
- By people with abnormal blood circulation in the brain
- By people experiencing pain in the stomach or intestine
- By individuals with a baclofen allergy
- By pregnant and nursing women
- Oral baclofen may be unsafe in individuals with liver disease or difficulty urinating
- Intrathecal baclofen may be unsafe for people with a history of heart problems, infections, or by those who are prone to autonomic dysreflexia
Even for those who are not restricted from using baclofen (see above), there may be risks and side effects with the use of this treatment. It is important to discuss these possibilities in detail with your health provider before using this treatment.
Risks and side effects of baclofen may include:
- Drowsiness, tiredness, or dizziness
- Muscle weakness
- Confusion
- Difficulty sleeping (insomnia)
- Interactions with other drugs such as antidepressants, sleeping pills, alcohol, and other medications
- Baclofen pumps are implanted surgically, which carries a risk of infection and other surgical risks
Because baclofen helps to relax the muscles, it may also have unintended effects on other medical problems that benefit from increased muscle tone. For example:
- It may further reduce the cough reflex in people who already have trouble coughing
- It may make it more difficult to walk, stand, or do other tasks requiring muscle strength and movement
- Baclofen pumps may make it more difficult for men to have erections, although this may be regained when reducing the dose or stopping treatment
In addition, stopping baclofen therapy abruptly can cause withdrawal. This can cause a variety of symptoms, including seizures, hallucinations, confusion, and fever. When baclofen is stopped, the dose of the medication should be gradually lowered over time before it can be stopped. It is important to follow the routine recommended by your health providers when stopping use of this medication.
Important considerations when treating spasticity
Although we often focus on the negative effects of spasticity, it can also have benefits. For example, spasticity in the legs can sometimes help people transfer more effectively or stand and walk. For this reason, when treatments like baclofen work the way they are supposed to, they can sometimes have negative effects, such as:
- Reduced functional abilities, such as the ability to transfer, stand, or walk
- Loss of health benefits of spasticity, such as better circulation and muscle strength
- Loss of spasticity as a warning sign of other health problems (such as infections or injuries below the level of injury)
The decision to treat spasticity needs to be made by you and your health team on a personal basis, taking into consideration function, symptoms, and the benefits and drawbacks of treatment.
Baclofen is a common treatment for spasticity after SCI. Both baclofen tablets and baclofen pumps are effective for reducing spasticity in people with SCI. As baclofen therapy requires careful dosing and monitoring, it is important to discuss with your health provider about whether this treatment option is suitable for you and how to use it appropriately.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, refer to SCIRE Community Evidence.
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Loh E, McIntyre A, Querée M, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0: p 1-135.
Available from: https://scireproject.com/evidence/spasticity/
Evidence for “What is baclofen?” is based on the following studies:
Oral baclofen:
[1] Chu V, Hornby T, Schmit B. Effect of antispastic drugs on motor reflexes and voluntary muscle contraction in incomplete spinal cord injury. Arch Phys Med Rehabil 2014;95:622-32.
[2] Nance P, Huff F, Martinez-Arizala A, Ayyoub Z, Chen D, Bian A, Stamler D. Efficacy and safety study of arbaclofen placarbil in patients with spasticity due to spinal cord injury. Spinal Cord 2011;49:974-80.
[3] Aydin G, Tomruk S, Keles I, Demir S, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil 2005;84:584-92.
[4] Duncan G, Shahani B, Young R. An evaluation of baclofen treatment for certain symptoms in patients with spinal cord lesions. A double-blind, cross-over study. Neurology 1976;26:441-6.
[5] Burke D, Gillies J, Lance J. An objective assessment of a gamma aminobutyric acid derivative in the control of spasticity. Proc Aust Assoc Neurol 1971;8:131-4.
[6] Dicpinigaitis P, Allusson V, Baldanti A, and Nalamati J. Ethnic and gender differences in cough reflex sensitivity. Respiration 2001;68:480-2.Dicpinigaitis P, Allusson V, Baldanti A, and Nalamati J. Ethnic and gender differences in cough reflex sensitivity. Respiration 2001;68:480-2.
[7] Veerakumar A, Cheng J, Sunshine A, Ye X, Zorowitz R, Anderson W. Baclofen dosage after traumatic spinal cord injury: a multi-decade retrospective analysis. Clin Neurol Neurosurg 2015;129:50-6.
[8] Nance P. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc 1994;17:150-6.
Intrathecal baclofen:
[1] Ordia J, Fischer E, Adamski E, Spatz E. Chronic intrathecal delivery of baclofen by a programmable pump for the treatment of severe spasticity. J Neurosurg 1996;85:452-7.
[2] Nance P, Schryvers O, Schmidt B, Dubo H, Loveridge B, Fewer D. Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci 1995;22:22-9.
[3] Coffey J, Cahill D, Steers W, Park T, Ordia J, Meythaler J, et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg 1993;78:226-32.
[4] Hugenholtz H, Nelson R, Dehoux E, Bickerton R. Intrathecal baclofen for intractable spinal spasticity-a double-blind cross-over comparison with placebo in 6 patients. Can J Neurol Sci 1992;19:188-95.
[5] Loubser P, Narayan R, Sandin K, Donovan W, Russell K. Continuous infusion of intrathecal baclofen: long-term effects on spasticity in spinal cord injury. Paraplegia 1991;29:48-64.
[6] Penn R, Savoy S, Corcos D, Latash M, Gottlieb G, Parke B et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989;320:1517-21.
[7] Boviatsis E, Kouyialis A, Korfias S, Sakas D. Functional outcome of intrathecal baclofen administration for severe spasticity. Clin Neurol Neurosurg 2005;107:289-95.
[8] Azouvi P, Mane M, Thiebaut J, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil 1996;77:35-9.
[9] Plassat R, Perrouin Verbe B, Menei P, Menegalli D, Mathe J, Richard I. Treatment of spasticity with intrathecal baclofen administration: Long-term follow-up review of 40 patients. Spinal Cord 2004;42:686-93.
[10] Zahavi A, Geertzen J, Middel B, Staal M, Rietman J. Long term effect (more than five years) of intrathecal baclofen on impairment, disability, and quality of life in patients with severe spasticity of spinal origin. J Neurol Neurosurg Psychi 2004;75:1553-7.
[11] Korenkov A, Niendorf W, Darwish N, Glaeser E, Gaab M. Continuous intrathecal infusion of baclofen in patients with spasticity caused by spinal cord injuries. Neurosurg Rev 2002;25:228-30.
[12] Broseta J, Garcia-March G, Sanchez-Ledesma M, Anaya J, Silva I. Chronic intrathecal baclofen administration in severe spasticity. Stereotact Funct Neurosurg 1990;54-55:147-53.
[13] Parke B, Penn R, Savoy S, Corcos D. Functional outcome after delivery of intrathecal baclofen. Arch Phys Med Rehabil 1989;70:30-2.
Other references:
Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine 2001; 26(24 Suppl):S146-S160.
Denys P, Mane M, Azouvi P, Chartier-Kastler E, Thiebaut JB, Bussel B. Side effects of chronic intrathecal baclofen on erection and ejaculation in patients with spinal cord lesions. Arch Phys Med Rehabil 1998; 79(5):494-496.
Dicpinigaitis PV, Dobkin JB, Reichel J. Typical versus cough-variant of asthma: differentiation by cough reflex sensitivity and the antitussive effect of zafirlukast. Eur Respir J. 2000; 16:525s.
Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl 1997; 6:S92-120.
Hinderer SR. The supraspinal anxiolytic effect of baclofen for spasticity reduction. Am J Phys Med Rehabil 1990; 69(5):254-258.
Jones ML, Leslie DP, Bilsky G, Bowman B. Effects of intrathecal baclofen on perceived sexual functioning in men with spinal cord injury. J Spinal Cord Med 2008; 31:97-102.
Kirshblum S. Treatment alternatives for spinal cord injury related spasticity. J Spinal Cord Med 1999; 22(3):199-217.
Knutsson E, Lindblom U, Martensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci 1974; 23(3):473-484.
Nance PW, Schryvers O, Schmidt B, Dubo H, Loveridge B, Fewer D. Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci 1995; 22:22-29.
Nance PW. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc 1994; 17(3):150-156.
Postma TJBM, Oenema D, Terpstra S et al. Cost analysis of the treatment of severe spinal spasticity with a continuous intrathecal baclofen infusion system. PharmacoEconomics 1999; 15(4):395-404.
CPS [Internet]. Ottawa (ON): Canadian Pharmacists Association; c2016 [cited 2017 Oct 10]. Available from: https://www.pharmacists.ca/products-services/ or http://www.myrxtx.ca. Also available in paper copy from the publisher.
Image credits
- Baclofen ball-and-stick model, ©Vaccinationist, CC BY-SA 4.0,
- National Institutes of Health, part of the United States Department of Health and Human ServicesBaclofen 20 mg oral tablet, CC0 1.0
- Intrathecal-pump-cartoon, ©R.E.B.E.L EM, CC BY-NC-ND 3.0.