Author: SCIRE Community Team | Reviewer: Patricia Mills | Published: 10 November 2017 | Updated: ~
Botulinum toxin injections may be used as a treatment after spinal cord injury (SCI). This page provides an overview of the use of botulinum toxin after SCI.
Key Points
- Botulinum toxin is a protein made by bacteria that can cause muscle weakness or paralysis.
- Very small doses of certain strains of botulinum toxin may be injected into muscles as a treatment for various medical conditions.
- Botulinum toxin injections are currently used to treat muscle spasticity and certain types of bladder problems after SCI.
- Research evidence supports that botulinum toxin is effective to reduce spasticity in muscles and to manage certain types of bladder problems after SCI.
A structure of a botulinum toxin molecule.1
Botulinum toxin is a protein produced by bacteria. Although this protein can be toxic to humans, injections of very small amounts of certain strains of botulinum toxin are used in medicine. Botulinum toxin is well-known by the trade names Botox, Dysport, and Xeomin as a cosmetic procedure for reducing wrinkles. However, it is also used as a treatment for various other medical conditions.
Botulinum toxin injections may be used after SCI to treat:
- Problematic spasticity that is located in specific muscles (widespread spasticity is usually treated with an oral medication instead)
- Overactive (reflex) bladder problems after SCI
Botulinum toxin injections into the bladder may also help to prevent autonomic dysreflexia that is triggered by bladder problems after SCI.
 Botulinum toxin is given with an injection into the affected spastic muscle or bladder.
Botulinum toxin is given with an injection into the affected spastic muscle or bladder.
The exact procedures and dose provided will be different for each person. Consult your health provider for further information about how botulinum toxin procedures may be done.
Multiple injections may be given in one session to ensure enough of the toxin reaches the muscle. After the injections, it may take up to a week for you to notice an effect. Exercise and stretching are usually recommended after the injection to enhance the effects of botulinum toxin.
Botulinum toxin injections are not permanent, and their effects wear off in usually around 3 months (in the case of muscle injections) to 6 months (in the case of bladder injections). Sessions are scheduled on an ongoing basis to maintain the effects of the treatment.
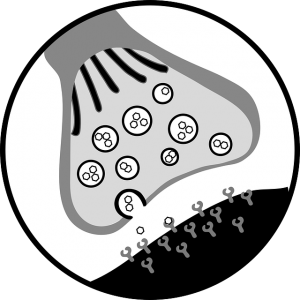
When botulinum toxin is injected into a muscle, it blocks the nerves to the muscles from releasing a chemical called acetylcholine. Acetylcholine makes muscles contract (tense). When its release is blocked, the muscles are unable to contract, causing weakness or paralysis. In a muscle with spasticity, botulinum toxin can help to decrease muscle spasms.

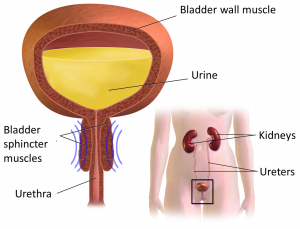
Botulinum toxin can be used to treat overactive (reflex) bladder problems for similar reasons. These bladder problems happen when the bladder muscle or the bladder sphincters spasm, preventing emptying or causing random emptying of urine (detrusor hyperreflexia). The injection of botulinum toxin into these muscles reduces muscle spasms, which may help to treat these problems.
Botulinum toxin is not suitable for everyone for medical reasons. It is also important to know that botulinum toxin treatments can be expensive depending on how your medications are funded. Consult your doctor for detailed information about whether this treatment is safe and appropriate for you.
Botulinum toxin should not be used in the following situations:

- By people with other neuromuscular disorders, such as myasthenia gravis
- By people who have an allergy to any of the injection ingredients
- By pregnant or nursing women
- In areas of infection
Botulinum toxin should be used with caution in the following situations:
- By people taking anticoagulants (blood thinners)
Additional precautions when botulinum toxin is used in the bladder:
- By people who have a current or multiple recent bladder or kidney infections (urinary tract infections)
- By people who are not willing or able to do clean intermittent catheterization or have a Foley catheter inserted. This is because there is a potential short term side effect of too much bladder muscle relaxation leading to an inability to fully empty the bladder (urinary retention) without a catheter.
Botulinum toxin injections are generally considered to have low risk of serious medical complications with their use. However, there are side effects and risks of this treatment that are important to discuss with your doctor. Side effects usually happen within the first few days after injection, but sometimes last longer. This is not a complete list; speak to your doctor for detailed information about botulinum toxin injections.
Risks and side effects of botulinum toxin injections may include:
- Muscle weakness – usually in the muscles that receive the injection, but may be generalized to other muscles, although this is a rare occurrence
- Long-term use may lead to loss of muscle size and bulk that happens when the muscles are not used (muscle atrophy)
Risks and side effects of botulinum toxin used for bladder problems include:
- Bladder and kidney infections (urinary tract infections)
- Blood in the urine
- An inability to fully empty the bladder (urinary retention)
For more information on urinary tract infections, check out our article here!
Risks and side effects related to injections of any kind:
In addition to the risks of botulinum toxin itself, injections of any kind may cause pain or tenderness, inflammation, changes to sensation, redness, infections, bleeding, bruising, light-headedness and fainting.
Important considerations when treating spasticity
Although we often focus on the negative effects of spasticity, it can also have benefits. For example, spasticity in the legs can sometimes help people transfer more effectively or stand and walk. For this reason, when treatments like botulinum toxin work the way they are supposed to, they can sometimes have negative effects, such as:
- Reduced functional abilities, such as the ability to transfer, stand, or walk
- Loss of health benefits of spasticity, such as better circulation, and muscle strength
- Loss of spasticity as a warning sign of other health problems (such as infections or injuries below the level of injury)
The decision to treat spasticity needs to be made by you and your health team on a personal basis, taking into consideration function, symptoms, and the benefits and drawbacks of treatment.
Botulinum toxin has been studied thoroughly as a treatment for spasticity in other conditions like stroke and brain injury. There is strong evidence to support that it is effective for treating spasticity in these conditions. Fewer studies have looked at how effective botulinum toxin injections are after SCI.
Spasticity
There is moderate evidence that botulinum toxin injections can be used to improve muscle spasticity in the injected muscle after SCI. It may also help to improve problems related to spasticity, such as pain, sleep disturbances, and walking problems.
Overactive (reflex) bladder problems
There is strong evidence that botulinum toxin injections are an effective treatment option for reducing the symptoms of overactive (reflex) bladder problems after SCI. This includes both:
- Injections into the bladder muscle to prevent leaking or incontinence
- Injections into the sphincter muscles to improve bladder emptying

The components of the urinary system.7
Autonomic dysreflexia and the spastic bladder
Some of the studies that have looked at treating bladder problems after SCI have also found that some participants also had fewer episodes of autonomic dysreflexia after treatment. This was thought to be because bladder problems triggered autonomic dysreflexia in these individuals. However, there is not enough evidence to use botulinum toxin as a direct treatment for preventing autonomic dysreflexia at this time.
See our article on Autonomic Dysreflexia for more information!
Botulinum toxin injections are a treatment option for spasticity and overactive (reflex) bladder problems after SCI. Botulinum toxin can be effective for reducing the symptoms related to these problems after SCI. It is important to discuss with your health provider about whether this treatment option is suitable for you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Project (Professional) “Bladder Management”, “Autonomic Dysreflexia”, and “Spasticity” chapters:
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Loh E, McIntyre A, Querée M, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0: p 1-135.
Available from: https://scireproject.com/evidence/rehabilitation-evidence/spasticity/
Hsieh J, McIntyre A, Iruthayarajah J, Loh E, Ethans K, Mehta S, Wolfe D, Teasell R. (2014). Bladder Management Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0: p 1-196.
Available from: https://scireproject.com/evidence/rehabilitation-evidence/bladder-management/
Krassioukov A, Blackmer J, Teasell RW, Eng JJ (2014). Autonomic Dysreflexia Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 35.
Available from: https://scireproject.com/evidence/rehabilitation-evidence/autonomic-dysreflexia/
Evidence for “Is botulinum toxin effective?” is based on the following studies:
Spasticity:
[1] Richardson D, Sheean G, Werring D, Desai M, Edwards S, Greenwood R et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychi 2000;69:499-506.
[2] Spiegl U, Maier D, Gonschorek O, Heyde C, Buhren V. Antispastic therapy with botulinum toxin type A in patients with traumatic spinal cord lesion. GMS Interdiscip Plast Reconstr Surg 2014;3:1-5.
[3] Bernuz B, Genet F, Terrat P, et al. Botulinum toxin effect on voluntary and stretch reflex-related torque produced by the quadriceps: An isokinetic pilot study. Neurorehabil Neural Repair 2012;26:542-7.
[4] Hecht M, Stolze H, uf dem B, Giess R, Treig T, Winterholler M et al. Botulinum neurotoxin type A injections reduce spasticity in mild to moderate hereditary spastic paraplegia–report of 19 cases. Mov Disord 2008;23:228-33.
[5] Al-Khodairy A, Gobelet C, Rossier A. Has botulinum toxin type A a place in the treatment of spasticity in spinal cord injury patients? Spinal Cord 1998;36:854-8.
Bladder problems:
Detrusor overactivity
[1] Mehta S, Hill D, McIntyre A, Foley N, Hsieh J, Ethans K et al. Meta-analysis of botulinum toxin A detrusor injections in the treatment of neurogenic detrusor overactivity after spinal cord injury. Arch Phys Med Rehabil 2013;94(8):1473-1481.
[2] Schurch B, de SM, Denys P, Chartier-Kastler E, Haab F, Everaert K et al. Botulinum toxin type is a safe and effective treatment for neurogenic urinary incontinence: Results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005;174(1):196-200.
[3] Grosse J, Kramer G, Jakse G. Comparing two types of botulinum-A toxin detrusor injections in patients with severe neurogenic detrusor overactivity: A case-control study. BJU International 2009;104:651-656.
[4] Schurch B, Denys Pierre, Kozma CM, Reese PR, Slaton T, Barron RL. Botulinum toxin A improves the quality of life of patients with neurogenic urinary incontinence. European urology 2007:52(3):850-859.
[5] Del Popolo G, Filocamo MT, Li Marzi V, Macchiarella A, Cecconi F, Lombardi G, Nicita G. Neurogenic detrusor overactivity treated with english botulinum toxin a: 8-year experience of one single centre. Eur Urol. 2008 May;53(5):1013-19.
[6] Giannantoni A, Meatini E, Del Zingaro M, Porena M. Six-year follow-up of Botulinum Toxin A intradetrosrial injections in patients with refractory neurogenic detrusor overactivity: Clinical and urodynamic results. European Urology 2009;55:705-712.
[7] Klaphajone J, Kitisomprayoonkul W, Sriplakit S. Botulinum toxin type A injections for treating neurogenic detrusor overactivity combined with low-compliance bladder in patients with spinal cord lesions. Arch Phys Med Rehabil 2005;86:2114-2118.
[8] Kuo H. Therapeutic effects of suburothelial injection of botulinum a toxin for neurogenic detrusor overactivity due to chronic cerebrovascular accident and spinal cord lesions. Urology 2006;67:232-236.
[9] Kuo H. Satisfaction with urethral injection of botulinum toxin A for detrusor sphincter dyssynergia in patients with spinal cord lesion. Neurourol Urodyn 2008;27:793-796.
[10] Pannek J, Gocking K, Bersch U. Long-term effects of repeated intradetrusor botulinum neurotoxin A injections on detrusor function in patients with neurogenic bladder dysfunction. BJU International 2009;104:1246-1250.
[11] Tow AM, Toh KL, Chan SP, Consigliere D. Botulinum toxin type A for refractory neurogenic detrusor overactivity in spinal cord injured patients in Singapore. Ann Acad Med Singapore 2007;36(1):11-17.
[12] Wefer B, Ehlken B, Bremer J, Burgdörfer H, Domurath B, Hampel C. Treatment outcomes and resource use of patients with neurogenic detrusor overactivity receiving botulinum toxin A (BOTOX®) therapy in Germany. World J Urol 2010;28(3):385-390.
[13] Akbar M, Abel R, Seyler TM, Bedke J, Haferkamp A, Gerner HJ et al. Repeated botulinum-A toxin injections in the treatment of myelodysplastic children and patients with spinal cord injuries with neurogenic bladder dysfunction. BJU Int 2007;100(3):639-645.
[14] Patki P, Hamid R, Shah PJ, Craggs M. Long-term efficacy of AMS 800 artificial urinary sphincter in male patients with urodynamic stress incontinence due to spinal cord lesion. Spinal Cord 2006;44(5):297-300.
[15] Herschorn S, Gajewski J, Ethans K, Corcos J, Carlson K, Bailly G et al. Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: A randomized, double-blind trial. J urology 2011;185(6):2229-2235.
[16] Abdel-Meguid T. Botulinum toxin-A injections into neurogenic overactive bladder—to include or exclude the trigone? A prospective, randomized, controlled trial. J urology 2010;184(6):2423-2428.
[17] Krhut J, Samal V, Nemec D, Zvara P. Intradetrusor versus suburothelial onabotulinumtoxinA injections for neurogenic detrusor overactivity: A pilot study. Spinal cord 2012;50(12):904-907.
Sphincter overactivity
[1] Kuo H. Therapeutic outcome and quality of life between urethral and detrusor botulinum toxin treatment for patients with spinal cord lesions and detrusor sphincter dyssynergia. Inter J Clin Prac 2013;67(10):1044-1049.
[2] Kuo H. Satisfaction with urethral injection of botulinum toxin A for detrusor sphincter dyssynergia in patients with spinal cord lesion. Neurourol Urodyn 2008;27:793-796.
[3] Tsai SJ, Ying TH, Huang YH, Cheng JW, Bih LI, Lew HL. Transperineal injection of botulinum toxin A for treatment of detrusor sphincter dyssynergia: Localization with combined fluoroscopic and electromyographic guidance. Arch Phys Med Rehabil 2009;90:832-836
[4] DeSeze M, Petit H, Gallien P, de Seze MP, Joseph PA, Mazaux JM et al. Botulinum a toxin and detrusor sphincter dyssynergia: A double-blind lidocaine-controlled study in 13 patients with spinal cord disease. Eur Urol 2002;42(1):56-62.
[5] Chen SL, Bih LI, Chen GD, Huang YH, You YH, Lew HL. Transrectal ultrasound-guided transperineal botulinum toxin A injection to the external urethral sphincter for treatment of detrusor external sphincter dyssynergia in patients with spinal cord injury. Arch Phys Med Rehabil 2010;91:340-344.
[6] Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: A prospective study in 24 spinal cord injury patients. J Urol 1996;155(3):1023-1029.
[7] Phelan MW, Franks M, Somogyi GT, Yokoyama T, Fraser MO, Lavelle JP et al. Botulinum toxin urethral sphincter injection to restore bladder emptying in men and women with voiding dysfunction. J Urol 2001;165(4):1107-1110.
Other references:
Alvares R, Silva,J, Barboza A, Monteiro R. Botulinum toxin A in the treatment of spinal cord injury patients with refractory neurogenic detrusor overactivity. International braz j urol 2010;36(6):732-737.
Bagi P, Biering-Sørensen F. Botulinum toxin A for treatment of neurogenic detrusor overactivity and incontinence in patients with spinal cord lesions. Scandinavian J Urol and nephrology 2004;38(6):495-498.
Caremel R, Courtois F, Charvier K, Ruffion A, Journel N. Side effects of intradetrusor botulinum toxin injections on ejaculation and fertility in men with spinal cord injury: Preliminary findings. BJU international 2012;109(11):1698-1702.
Chang E, Ghosh N, Yanni D, Lee S, Alexandru D, Mozaffar T. A Review of Spasticity Treatments: Pharmacological and Interventional Approaches. Crit Rev Phys Rehabil Med. 2013;25(1-2):11-22.
Chen G, Liao L. Injections of botulinum toxin A into the detrusor to treat neurogenic detrusor overactivity secondary to spinal cord injury. Intern Urol Nephrol 2011;43(3):655-662.
Chen S, Kuo H. Therapeutic outcome and patient adherence to repeated onabotulinumtoxinA detrusor injections in chronic spinal cord-injured patients and neurogenic detrusor overactivity. Journal of the Formosan Medical Association 2013.
Cho YS, Kim KH. Botulinum toxin in spinal cord injury patients with neurogenic detrusor overactivity. J Exerc Rehabil. 2016 Dec 31;12(6):624-631.
CPS [Internet]. Ottawa (ON): Canadian Pharmacists Association; c2016 [cited 2017 Nov 2]. Available from: https://www.pharmacists.ca/products-services/ or http://www.myrxtx.ca. Also available in paper copy from the publisher.
Del Popolo G, Filocamo M, Li Marzi V, Macchiarella A, Cecconi F, Lombardi G, et al. Intermittent self-catheterization habits and opinion on aspetic VaPro catheter in French neurogenic bladder population. Spinal Cord 2012;50(11):853-858.
Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139(5):919-922.
Dykstra DD, Sidi AA. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: A double-blind study. Arch Phys Med Rehabil 1990;71(1):24-26.
Ehren I, Volz D, Farrelly E, Berglund L, Brundin L, Hultling C et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: A randomised, placebo-controlled, double-blind study. Scand J Urol Nephrol 2007;41(4):335-340.
Fried G & Fried K. Spinal cord injury and use of botulinum toxin in reducing spasticity. Phys Med Rehabil Clin N Am 2003;14:901-10.
Fried GW, Fried KM. Spinal cord injury and use of botulinum toxin in reducing spasticity. Phys Med Rehabil Clin N Am. 2003 Nov;14(4):901-10. Review.
Game X, Chartier-Kastler E, Ayoub N, Even-Schneider A, Richard F, Denys P. Outcome after treatment of detrusor-sphincter dyssynergia by temporary stent. Spinal Cord 2008; 46:74-77.
Grosse J, Kramer G, Jakse G. Comparing two types of botulinum-A toxin detrusor injections in patients with severe neurogenic detrusor overactivity: A case-control study. BJU International 2009; 104:651-656.
Haar GT, Dyson M, Oakley EM. The use of ultrasound by physiotherapists in Britain, 1985. Ultrasound in Med & Biol 1987. 13(10): 659-663.
Haferkamp A, Schurch B, Reitz A, Krengel U, Grosse J, Kramer G. Lack of ultrastructural detrusor changes following endoscopic injection of botulinum toxin type A in overactive neurogenic bladder. European urology 2004;46(6):784-791.
Hajebrahimi S, Altaweel W, Cadoret J, Cohen E, Corcos J. Efficacy of botulinum-A toxin in adults with neurogenic overactive bladder: Initial results. Canadian J Urol 2005;12:2543-2546.
Hikita K, Honda M, Kawamoto B, Panagiota T, Inoue S, Hinata N. Botulinum toxin type A injection for neurogenic detrusor overactivity: Clinical outcome in Japanese patients. International J Urol 2013;20(1):94-99.
Hori S, Patki P, Attar K, Ismail S, Vasconcelos J, Shah P. Patients’ perspective of botulinum toxin-A as a long-term treatment option for neurogenic detrusor overactivity secondary to spinal cord injury. BJU International 2009;104,:216-220.
Karsenty G, Chartier-Kastler E, Mozer P, Even-Schneider A, Denys P, Richard F. A novel technique to achieve cutaneous continent urinary diversion in spinal cord-injured patients unable to catheterize through native urethra. Spinal Cord 2008;46(4):305-310.
Karsenty G, Reitz A, Lindemann G, Boy S, Schurch B. Persistence of therapeutic effect after repeated injections of botulinum toxin type A to treat incontinence due to neurogenic detrusor overactivity. Urology 2006;68(6):1193-1197.
Kaviani A, Khavari R. Disease-Specific Outcomes of Botulinum Toxin Injections for Neurogenic Detrusor Overactivity. Urol Clin North Am. 2017 Aug;44(3):463-474.
Kuo H. Satisfaction with urethral injection of botulinum toxin A for detrusor sphincter dyssynergia in patients with spinal cord lesion. Neurourol Urodyn 2008;27:793-796.
Kuo H. Therapeutic outcome and quality of life between urethral and detrusor botulinum toxin treatment for patients with spinal cord lesions and detrusor sphincter dyssynergia. Inter J Clin Prac 2013;67(10):1044-1049.
Kuo HC, Liu SH. Effect of repeated detrusor onabotulinumtoxinA injections on bladder and renal function in patients with chronic spinal cord injuries. Neurourol Urodyn 2011;30:1541–1545.
Lui J, Sarai M, Mills PB. Chemodenervation for treatment of limb spasticity following spinal cord injury: a systematic review. Spinal Cord. 2015 Apr;53(4):252-64. doi: 10.1038/sc.2014.241. Epub 2015 Jan 13. Review.
Lui J, Sarai M, Mills PB. Chemodenervation for treatment of limb spasticity following spinal cord injury: a systematic review. Spinal Cord. 2015 Apr;53(4):252-64. doi: 10.1038/sc.2014.241. Epub 2015 Jan 13. Review.
Marciniak C, Rader L, Gagnon C. The use of botulinum toxin for spasticity after spinal cord injury. Am J Phys Med Rehabil. 2008 Apr;87(4):312-7; quiz 318-20, 329.
Mascarenhas F, Cocuzza M, Gomes C, Leão N. Trigonal injection of botulinum toxin‐A does not cause vesicoureteral reflux in neurogenic patients. Neurourol Urodyn 2008;27(4):311-314.
Ni J, Wang X, Cao N, Si J, Gu B. Is repeat Botulinum Toxin A injection valuable for neurogenic detrusor overactivity-A systematic review and meta-analysis. Neurourol Urodyn. 2017 Jul 26. doi: 10.1002/nau.23354. [Epub ahead of print]
Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol 2010; 55(1):8-14.
Petit H, Wiart L, Gaujard E, Le BF, Ferriere JM, Lagueny A et al. Botulinum A toxin treatment for detrusor-sphincter dyssynergia in spinal cord disease. Spinal Cord 1998;36(2):91-94.
Reitz A, Denys P, Fermanian C, Schurch B, Comperat E, Chartier-Kastler E. Do repeat intradetrusor botulinum toxin type a injections yield valuable results? Clinical and urodynamic results after five injections in patients with neurogenic detrusor overactivity. Euro Urol 2007;52(6):1729-1735.
Reitz A, Stohrer M, Kramer G, Del Popolo G, Chartier-Kastler E, Pannek J et al. European experience of 200 cases treated with Botulinum-A Toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. European Urology 2004;45:510-515.
Richardson D, Edwards S, Sheean GL, Greenwood RJ, Thompson AJ. The effect of botulinum toxin on hand function after incomplete spinal cord injury at the level of C5/6: a case report. Clin Rehabil 1997; 11(4):288-292.
Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J Urol 2000;164(3 Pt 1):692-697.
Image credits
- Botulinum toxin 3BTA ©Clr324, CC0 1.0
- Injection ©priyanka, CC BY 3.0 US
- Synapse ©Clker-Free-Vector-Images, CC0 1.0
- Injection ©Vectors Point, CC BY 3.0 US
- Pregnancy botox risk ©waldryano, CC0 1.0
- Pain botox ©3dman_eu, CC0 1.0
- Modified from: Urinary Sphincter ©BruceBlaus, CC BY-SA 4.0