Author: SCIRE Community Team | Reviewer: Chris S. Bailey | Published: 22 January 2018 | Updated: ~
Neuroprotection describes a wide range of treatments that aim to protect the spinal cord from further damage in the hours to weeks after an injury first happens. This page explains what neuroprotection is and outlines what the most promising treatments are right now.
Key Points
- Spinal cord damage can worsen in the days to weeks after a spinal cord injury (SCI) because of processes like inflammation and lack of blood flow. This is called secondary injury.
- Neuroprotection is the use of medical treatments early after the initial injury to help protect the spinal cord from further damage.
- A wide range of medications has been suggested as neuroprotection, including the steroid methylprednisolone. Other types of treatments, such as cooling the body, have also been studied.
- Neuroprotection treatments are controversial at this time because there is not enough evidence to support using most treatments and there is disagreement amongst experts about how to interpret research findings for using methylprednisolone.
- Neuroprotection is an emerging area of research and many current studies are ongoing.
Neuroprotection is the use of medical treatments to protect the spinal cord from further injury in the hours to weeks after a spinal cord injury first happens. The term neuroprotection is used to describe a wide range of mainly experimental treatments that aim to reduce damage known as secondary injury.
Secondary injury
There are two main phases of damage that happen after a spinal cord injury, primary injury and secondary injury. The damage that is directly caused by the initial injury is called primary injury. Primary injury happens when the spinal cord is bruised, compressed, pulled or torn, which directly injures the nerve cells and spinal cord tissues.
As the body responds to the initial injury, several bodily processes can happen, which further damage the spinal cord. This is known as secondary injury. Secondary injury is caused by several processes, such as inflammation, lack of blood flow (ischemia), and build-up of damaging chemicals, which can worsen the original injury.
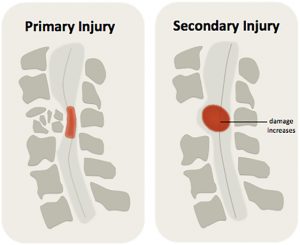
Secondary injury can worsen the spinal cord injury, expanding the size of the injury and leading to further loss of function.1
Secondary injury processes are responsible for expanding the size of the injury in the days to weeks afterwards and causing further loss of function. They can also make it harder for the body to heal.
Neuroprotection is intended to help protect the spinal cord from further damage, it is not intended to heal or repair already damaged tissues (this is called regeneration). Neuroprotective treatments are usually given as early as possible after injury, which is usually within the first 24 hours after injury or at the time of the first surgery.
Neuroprotection is also used to help protect against further nerve damage in other neurological conditions, such as brain injuries and degenerative diseases like amyotrophic lateral sclerosis (ALS).
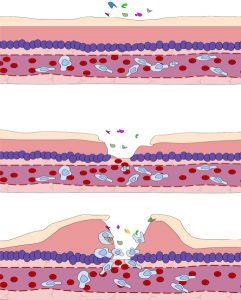
During inflammation, special immune cells help get rid of foreign substances. This can lead to localized swelling.2
Inflammation and swelling
Inflammation is a natural bodily process where cells from the immune system (such as white blood cells) are brought to the site of an injury or illness. These cells remove harmful bacteria and dead cells from the area to help with healing.
Some inflammation is needed for healing. However, when it is excessive or continues for a long time, it can be damaging. Inflammation can end up breaking down healthy nerve cells (neurons), which makes the injury worse. Inflammation and other factors can also contribute to swelling of the spinal cord near the injury. Swelling can compress the spinal cord even more, damaging nerve cells and impairing blood flow.
Lack of blood flow
When the spinal cord is injured, the small blood vessels that provide oxygen and nutrients to the cord are also injured. If the blood vessels are torn, they may bleed within the spinal cord causing a bruise (called haemorrhage), which can increase the pressure on the spinal tissues and cause damage.
If the blood vessels are compressed or if there is not enough whole body blood pressure (which happens as a part of neurogenic shock), they are also unable to maintain adequate blood flow to the cord. This is called ischemia (pronounced ‘iss-kee-mee-ah’). A lack of blood flow means that oxygen or nutrients cannot reach the tissues, which can damage or kill healthy cells. Ischemia can become severe within hours after the injury and can damage nerve cells and surrounding tissues, causing the injury to get bigger.
Build-up of damaging chemicals
Damaged nerve cells can cause the release of chemical compounds like glutamate. Glutamate is a neurotransmitter that stimulates nerve cells to fire. However, too much glutamate can overstimulate the cells, causing calcium to build up inside them, which damages and kills healthy cells. This is called excitotoxicity. Excitotoxicity is a major cause of secondary damage after SCI.
Inflammation, excitotoxicity and cell damage can release by-products called free radicals. Free radicals are molecules that are unstable and highly reactive. Free radicals can damage DNA, the fragile outer membranes, and other parts of cells.
Nerve cell death
Cells can die because of damage or injury (called necrosis) or because the body triggers the cells to self-destruct through a process called apoptosis.
Apoptosis is sometimes called ‘programmed cell death’ because it is a natural part of how the body gets rid of cells it doesn’t need.
Under normal circumstances, apoptosis is carefully controlled by the body. However, certain conditions can trigger apoptosis when it is not needed. For example, damage to parts of the cell or chemical imbalances around or within the cell can trigger apoptosis.
After an SCI, secondary injury processes like excitotoxicity, inflammation, and the release of free radicals can trigger apoptosis. This can affect both nerve cells (neurons) and supporting cells like the cells that maintain myelin. This can even happen far away from the injury. Cell apoptosis after SCI expands the area of damage by killing previously healthy neurons and supporting cells.
‘Neuroprotection’ refers to a wide range of different treatments, including medications and other treatments like cooling the body that aim to reduce secondary injury. It is sometimes used to describe surgical decompression and blood pressure control after SCI, although these treatments are not discussed on this page.
While there are many different neuroprotective treatment options being explored, below we briefly outline the most promising treatments currently being studied.
Most neuroprotection treatments are experimental
Currently, there are no widely accepted treatments used for neuroprotection. With the exception of methylprednisolone (see below), most of the potential treatments are currently being tested in research studies. Because of the experimental nature of these treatments, most of the treatments outlined below are not available for most people or used outside of research settings.
For details about the progress of ongoing clinical trials, please visit ClinicalTrials.gov.
Steroids (Methylprednisolone)
The steroid medication Methylprednisolone is the most well-known neuroprotective treatment. It is also the only treatment that is currently used outside of research studies. High doses of methylprednisolone may affect many different aspects of secondary injury, including reducing inflammation and damage from free radicals that are thought to help prevent cells from dying after injury.
Three large-scale clinical trials were completed during 1980’s and 1990’s, which established methylprednisolone as a standard treatment that all patients with SCI should receive. However, later debate among experts criticized conclusions made in these studies and questioned the value of methylprednisolone as a treatment. It has been argued that methylprednisolone has shown only limited benefits for recovery, but well-established risk of side effects like infections.
At this time, methylprednisolone continues to be a controversial treatment among experts. It may be used in some clinical settings in certain groups of people with SCI.
Riluzole
Riluzole is a drug that blocks sodium from entering into nerve cells, which may help to reduce excitotoxicity. Riluzole is currently used to treat the degenerative neurological condition amyotrophic lateral sclerosis (ALS).
Animal studies have shown that Riluzole is effective as a neuroprotection treatment in rats with SCI. One preliminary study supported that Riluzole is safe and has potential to provide neuroprotection after SCI in humans. Currently, a large-scale clinical trial is in progress to determine whether Riluzole is effective as neuroprotection after SCI.
Minocycline
Minocycline is an antibiotic medication that is most commonly used to treat bacterial infections. Minocycline may also have neuroprotective effects after SCI. Animal studies have shown that minocycline may suppress immune cells involved in inflammation, cell death and the release of damaging chemicals. An early clinical trial has shown that IV minocycline is safe for use after acute SCI, and has potential as neuroprotection in people with incomplete SCI. Currently, a large-scale study is ongoing to investigate whether minocycline is effective as neuroprotection after SCI.
Cooling the body (therapeutic hypothermia)
Cooling the body, known as therapeutic hypothermia, is a treatment that involves reducing the temperature of the body to help protect against further damage. The body is cooled by inserting a cooling catheter into a blood vessel, which cools blood moving through circulation by a few degrees. Reducing body temperature slows metabolism, which can reduce inflammation and minimize further damage. This procedure is currently used to help prevent neurological damage after cardiac arrest.
A small preliminary study has shown that therapeutic hypothermia is safe and has potential as neuroprotection after SCl. Currently, a clinical trial is underway to determine whether spinal cord cooling is effective as neuroprotection for SCI in humans.
Cerebrospinal fluid drainage
Cerebrospinal fluid (CSF) drainage is a technique that involves inserting a small catheter through the coverings of the spinal cord to drain a small amount of the fluid that surrounds the spinal cord and brain (called cerebrospinal fluid). Draining the cerebrospinal fluid is thought to help relieve pressure and reduce further injury to the spinal cord.
Animal studies have shown that cerebrospinal fluid drainage may help improve spinal cord blood flow when combined with careful blood pressure management. One small research study has shown that cerebrospinal fluid drainage was safe for use in people with acute SCI. A larger study has been completed to determine whether cerebrospinal fluid drainage is effective as neuroprotection after SCI. However, the results have not yet been published.
Magnesium
Magnesium has been proposed as a neuroprotective treatment because it can help to reduce excitotoxicity and inflammation. Animal studies have shown benefits for magnesium as neuroprotection.
Cethrin (VX-210)
Cethrin (VX-210) is a medication that reduces the effects of Rho, a protein that is present in inflammation. Rho causes damage to nerve cells and prevents nerve cell regrowth. Cethrin reverses the action of Rho, which may help protect nerve cells and allow neurons to regrow. An early study has shown that Cethrin is safe. A clinical trial testing whether it is effective has been completed with the results pending publication.
Other treatments being studied
A wide range of other treatments have been or are currently being studied. These include:
- Fibroblast growth factor
- Hepatocyte growth factor
- Erythropoetin (EPO)
- GM-1 Ganglioside (Sygen)
- Granulocyte-colony stimulating factor (G-CSF)
- Thyrotropin-releasing hormone (TRH)
- Glibenclamide or glyburide
- Special diets such as intermittent fasting
Neuroprotective treatments that are not effective
Research evidence on the following treatments has suggested that they are not effective for use as neuroprotection after SCI. These include:
- Naloxone
- Tirilazad mesylate (was not more effective than methylprednisolone, but had more side effects)
- Nimodipine
- Gacyclidine
Secondary injury is a complicated process that scientists are still working to understand. While there are many promising treatments currently being studied, there are no widely accepted treatments so far. Neuroprotection is a relatively new area of study compared to other aspects of medicine and there are a number of other factors that makes neuroprotection difficult to study.
Testing experimental treatments is lengthy and expensive
 Most neuroprotective treatments are experimental and have not been tested in humans. This means that each treatment must undergo vigorous testing to determine if it is safe and effective. There are several phases of study that must be done. Usually, research studies are tested on animals first, and then typically go through at least three phases of human studies (Phase I, II, and III clinical trials) before a treatment can be determined to be safe and effective for real world use. These trials can cost millions of dollars and take several years to complete.
Most neuroprotective treatments are experimental and have not been tested in humans. This means that each treatment must undergo vigorous testing to determine if it is safe and effective. There are several phases of study that must be done. Usually, research studies are tested on animals first, and then typically go through at least three phases of human studies (Phase I, II, and III clinical trials) before a treatment can be determined to be safe and effective for real world use. These trials can cost millions of dollars and take several years to complete.
Designing and conducting high quality studies is very difficult
 It is also very difficult to design and carry out high quality studies. There are many factors relevant to the design and how the study findings are analyzed that can affect the study’s findings. This is why many previous research studies (such as those done on methylprednisolone), are controversial even amongst experts.
It is also very difficult to design and carry out high quality studies. There are many factors relevant to the design and how the study findings are analyzed that can affect the study’s findings. This is why many previous research studies (such as those done on methylprednisolone), are controversial even amongst experts.
In addition, neuroprotection treatments are usually given immediately after the injury during a highly stressful and confusing period. It can be challenging for patients and their families to determine whether they want to take part in a study and give their consent to participate.
Promising experimental treatments often do not translate into effective treatments
 Unfortunately, many treatments that are shown to be effective in animal studies do not go on to show the same effects in the real world scenarios involved in clinical trials. Translating research into effective treatments is a complex process with many steps that are undertaken to ensure that treatments are safe and effective.
Unfortunately, many treatments that are shown to be effective in animal studies do not go on to show the same effects in the real world scenarios involved in clinical trials. Translating research into effective treatments is a complex process with many steps that are undertaken to ensure that treatments are safe and effective.
Because secondary injury is so complex, some researchers believe that it is unlikely that one single treatment will be discovered that will completely “protect” the spinal cord from further damage. Research may lead clinicians to several different treatment options that may be used together or in different situations to help protect the spinal cord from further damage.
Neuroprotection is the use of medical treatments early after the initial injury to help protect the spinal cord from further damage caused by secondary injury.
There are many different treatments being studied for use as neuroprotection. The steroid methylprednisolone is the most well known treatment, but its use is controversial among experts. Most neuroprotective treatments are currently being investigated in research studies.
Neuroprotection after SCI is an emerging area of research and clinical care that we will learn more about as research findings arise in the next few years.
Parts of this page have been adapted from the SCIRE Project (Professional) “Neuroprotection during the Acute Phase of Spinal Cord Injury” Chapter:
Mullen E, Mirkowski M, Hsieh JTC, Bailey C, McIntyre A, Teasell RW. (2015). Neuroprotection during the Acute Phase of Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Research Evidence. Version 5.0: p 1-42.
Available from: https://scireproject.com/evidence/acute-evidence/neuroprotection-during-acute-phase-of-spinal-cord-injury/
Evidence for “Steroids (Methylprednisolone)”
Alibai E, Zand F, Rahimi A, Rezaianzadeh A. Erythropoietin plus methylprednisolone or methylprednisolone in the treatment of acute spinal cord injury: a preliminary report. Acta Med Iran 2014; 52(4):275-279.
Amar AP, Levy ML. Surgical controversies in the management of spinal cord injury. J Am Coll Surgeons 1999; 188(5):550-566.
Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the second national acute spinal cord injury study. New Engl J Med 1990; 322(20):1405-1411.
Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997; 277(20):1597-1604.
Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up: results of the third national acute spinal cord injury randomized controlled trial. J Neurosurg 1998; 89(5):699-706.
Chikuda H, Yasunaga H, Takeshita K, et al. Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg Med J 2014; 31(3):201-206.
Christie SD, Comeau B, Myers T, Sadi D, Purdy M, Mendez I. Duration of lipid peroxidation after acute spinal cord injury in rats and the effect of methylprednisolone: laboratory investigation. Neurosurg Focus 2008; 25(5).
George ER, Scholten DJ, Buechler CM, et al. Failure of methylprednisolone to improve the outcome of spinal cord injuries. Am Surgeon 1995; 61(8):659-664.
Gerhart KA, Johnson RL, Menconi J, Hoffman RE, Lammertse DP. Utilization and effectiveness of methylprednisolone in a population-based sample of spinal cord injured persons. Paraplegia 1995; 33(6):316-321.
Gerndt SJ, Rodriguez JL, Pawlik JW, et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma 1997; 42(2):279-284.
Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg 1992; 76(1):13-22.
Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine 2009; 34(20):2121-2124.
Lee BH, Lee KH, Yoon DH, et al. Effects of methylprednisolone on the neural conduction of the motor evoked potentials in spinal cord injured rats. J Korean Med Sci 2005; 20(1):132-138.
Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine 2001; 26(4):426-430.
Prendergast MR, Saxe JM, Ledgerwood AM, et al. Massive steroids do not reduce the zone of injury after penetrating spinal cord injury. J Traum 1994; 37(4):576-580.
Rasool T, Wani MA, Kirmani AR, et al. Role of methylprednisolone in acute cervical cord injuries. Indian J Surg 2004; 66(3):156-159.
Suberviola B, González-Castro A, Llorca J, Ortiz-Melón F, Miñambres E. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury 2008; 39(7):748-752.
Vaquero J, Zurita M, Oya S, Aguayo C, Bonilla C. Early administration of methylprednisolone decreases apoptotic cell death after spinal cord injury. Histol Histopathol 2006; 21(10-12):1091-1102.
Xiong M, Chen S, Yu H, Liu Z, Zeng Y, Li F. Neuroprotection of erythropoietin and methylprednisolone against spinal cord ischemia-reperfusion injury. J Huazhong Univ Sci 2011; 31(5):652-656.
Zhuang C, Wang L, Xu Y. Early methylprednisolone impact treatment for sensory and motor function recovery in patients with acute spinal cord injury: a self-control study. Neural Regen Res 2008; 3(5):577-580.
Evidence for “Riluzole”
Fehlings MG, Nakashima H, Nagoshi N, Chow DS, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016 Jan;54(1):8-15.
Grossman RG, Fehlings MG, Frankowski RF, et al. A prospective, multicenter, Phase i matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotraum 2014; 31(3):239-255.
Satkunendrarajah K, Nassiri F, Karadimas SK, Lip A, Yao G, Fehlings MG. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol. 2016 Feb;276:59-71.
Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg 2001; 94(2 Suppl):245-256.
Wilson JR, Fehlings MG. Riluzole for acute traumatic spinal cord injury: a promising neuroprotective treatment strategy. World Neurosurg 2014; 81(5-6):825-829.
Evidence for “Minocycline”
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012; 135(Pt 4):1224-1236.
Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol 2004; 3(12):744-751.
Evidence for “Cooling the body (therapeutic hypothermia)”
Kwon BK, Mann C, Sohn HM et al. , Hypothermia for spinal cord injury. Spine J. 2008, 8, 6:859–874.
Lo TP Jr, Cho KS, Garg MSet al. , Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol . 2009, 514, 5:433–448.
Levi AD, Green BA, Wang MYet al. , Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma . 2009, 26, 3:407–415.
Evidence for “Cerebrospinal fluid drainage”
Kwon BK, Curt A, Belanger LMet al. , Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. Journal Neurosurg Spine . 2009, 10, 3:181–193.
Martirosyan NL, Kalani MY, Bichard WD, et al. Cerebrospinal fluid drainage and induced hypertension improve spinal cord perfusion after acute spinal cord injury in pigs. Neurosurgery 2015;76(4):461-469
Evidence for “Magnesium”
Kaptanoglu E, Beskonakli E, Okutan O, Selcuk Surucu H, Taskin Y. Effect of magnesium sulphate in experimental spinal cord injury: evaluation with ultrastructural findings and early clinical results. J Clin Neurosci 2003a;10(3): 329-334.
Kaptanoglu E, Beskonakli E, Solaroglu I, Kilinc A, Taskin Y. Magnesium sulfate treatment in experimental spinal cord injury: emphasis on vascular changes and early clinical results. Neurosurg Rev 2003b;26(4):283-287.
Evidence for “Cethrin (VX-210)”
Fehlings MG, Theodore N, Harrop J, et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotraum 2011; 28(5):787-796.
McKerracher L, Guertin P. Rho as a target to promote repair: translation to clinical studies with cethrin. Curr Pharm Design 2013; 19(24):4400-4410.
Evidence for “Other treatments”
Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen® multicenter acute spinal cord injury study. Spine 2001c; 26(24 Suppl):S87-S100.
Geisler FH, Dorsey FC, Coleman WP. GM-1 ganglioside in human spinal cord injury. J Neurotraum 1992; (9 Suppl 2):S517-530.
Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury – a randomized, placebo-controlled trial with GM-1 ganglioside. New Engl J Med 1991; 324(26):1829-1838.
Kamiya K, Koda M, Furuya T, et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: a comparison with high-dose methylprednisolone as a historical control. Eur Spine J 2014; 24(5):963-967.
Khorasanizadeh M, Eskian M, Vaccaro AR, Rahimi-Movaghar V. Granulocyte Colony-Stimulating Factor (G-CSF) for the Treatment of Spinal Cord Injury. CNS Drugs. 2017 Nov;31(11):911-937.
Kitamura K, Fujiyoshi K, Yamane J, et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One 2011b;6:e27706.
Kitamura K, Iwanami A, Fujiyoshi K, et al. Recombinant human hepatocyte growth factor promotes functional recovery after spinal cord injury. PLoS One 2011a;6(11):e27706.
Kitamura K, Iwanami A, Nakamura M, et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res 2007;85(11):2332-2342.
Pitts LH, Ross A, Chase GA, Faden AI. Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotraum 1995; 12(3):235-243.
Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle 2005; 4(12):1753-1757.
Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA 2001; 98(7):4044-4049.
Tadie M, Gaviria M, Mathe J, et al. Early care and treatment with a neuroprotective drug, gacyclidine, in patients with acute spinal cord injury. Rachis 2003; 15:363-376.
Takahashi H, Yamazaki M, Okawa A, et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. Eur Spine J 2012; 21(12):2580-2587.
Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci 1999;19(16):7037-7047.
Evidence for “Neuroprotective treatments that are not effective”
Flamm ES, Young W, Collins WF, Piepmeier J, Clifton GL, Fischer B. A phase I trial of naloxone treatment in acute spinal cord injury. J Neurosurg 1985; 63(3):390-397.
Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the second national acute spinal cord injury study. New Engl J Med 1990; 322(20):1405-1411.
Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997; 277(20):1597-1604.
Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up: results of the third national acute spinal cord injury randomized controlled trial. J Neurosurg 1998; 89(5):699-706.
Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord, 2000;38(2):71-76.
Tadie M, Gaviria M, Mathe J, et al. Early care and treatment with a neuroprotective drug, gacyclidine, in patients with acute spinal cord injury. Rachis, 2003;15:363-376.
Other references
Ahuja CS, Martin AR, Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016 May 27;5. pii: F1000 Faculty Rev-1017.
Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord 2015; 53(1):14-18.
Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 2001; 103(1):203-218.
Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem 2007; 14(11):1189-1197.
Fehlings MG, Baptiste DC. Current status of clinical trials for acute spinal cord injury. Injury 2005; 36(Suppl 2):SB113-SB122.
Geisler FH, Coleman WP, Grieco G, Poonian D. Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine 2001a; 26(24 Suppl):S68-S86.
Geisler FH, Coleman WP, Grieco G, Poonian D. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine 2001b; 26(24 Suppl):S58-S67.
Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004 Jan;1(1):80-100.
Hall ED. Drug development in spinal cord injury: what is the FDA looking for? J Rehabil Res Dev 2003; 40(4 Suppl 1):81-91.
Heary RF, Vaccaro AR, Mesa JJ, et al. Steroids and gunshot wounds to the spine. Neurosurgery 1997; 41(3):576-584.
Hilton BJ, Moulson AJ, Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci Lett. 2017 Jun 23;652:3-10.
Kan EM, Ling EA, Lu J. Stem cell therapy for spinal cord injury. Curr Med Chem 2010; 17(36):4492-4510.
Kavanagh RJ, Kam PCA. Lazaroids: efficacy and mechanism of action of the 21-aminosteroids in neuroprotection. Brit J Anaesth 2001; 86(1):110-119.
Kigerl K, Popovich P. Drug evaluation: ProCord – a potential cell-based therapy for spinal cord injury. IDrugs 2006; 9(5):354-360.
Levy ML, Gans W, Wijesinghe HS, SooHoo WE, Adkins RH, Stillerman CB. Use of methylprednisolone as an adjunct in the management of patients with penetrating spinal cord injury: outcome analysis. Neurosurgery 1996; 39(6):1141-1149.
Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotraum 2000; 17(10):871-890.
Merry WH, Cogbill TH, Annis BL, Lambert PJ. Functional outcome after incomplete spinal cord injuries due to blunt injury. Injury 1996; 27(1):17-20.
Meurer WJ, Barsan WG. Spinal cord injury neuroprotection and the promise of flexible adaptive clinical trials. World Neurosurg. 2014 Sep-Oct;82(3-4):e541-6.
Mocchetti I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol Life Sci 2005; 62(19-20):2283-2294.
Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000; 38(2):71-76.
Pollard ME, Apple DF. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine 2003; 28(1):33-38.
Poynton AR, O’Farrell DA, Shannon F, Murray P, McManus F, Walsh MG. An evaluation of the factors affecting neurological recovery following spinal cord injury. Injury 1997; 28(8):545-548.
Santamaria AJ, Guest JD. The current status of neuroprotection for spinal cord injury. In: Weidner N., Rupp R, Tansey K (eds) Neurological aspects of spinal cord injury. 2017 Springer, Cham.
Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res 2015;218:15-54.
Simard JM, Tsymbalyuk O, Ivanov A, et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest 2007;117(8):2105–2113.
Tator CH. Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery 1998; 42(4):696-708.
Teng YD, Mocchetti I, Wrathall JR. Basic and acidic fibroblast growth factors protect spinal motor neurones in vivo after experimental spinal cord injury. Eur J Neurosci 1998;10(2):798-802.
Wallace MC, Tator CH, Frazee P. Relationship between posttraumatic ischemia and hemorrhage in the injured rat spinal cord as shown by colloidal carbon angiography. Neurosurgery 1986; 18(4):433-439.
Waxman SG. Demyelination in spinal cord injury. J Neurol Sci 1989; 91(1-2):1-14.
Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res 2012; 37(6):1230-1244.
Image credits:
- ‘Figure 3 – There are two phases of injury after damage to the spinal cord’ from: O’Higgins M, Badner A and Fehlings M (2017) What Is Spinal Cord Injury? Front Young Minds. 5:17. doi: 10.3389/frym.2017.00017. (CC BY 3.0)
- Immune response ©Nason vassiliev, CC BY-SA 4.0
- Expensive watch ©Vectors Point, CC BY 3.0 US
- Overworked ©Luis Prado, CC BY 3.0 US
- Explosion in lab ©Gan Khoon Lay, CC BY 3.0 US
Disclaimer: This document does not provide medical advice. This information is provided for educational purposes only. Consult a qualified health professional for further information or specific medical advice. The SCIRE Project, its partners and collaborators disclaim any liability to any party for any loss or damage by errors or omissions in this publication.
Authors: SCIRE Community Team | Reviewer: Bonnie Nybo | Published: 18 January 2018 | Updated: 16 October 2024
Key Points
- Most people with SCI experience some bladder changes after injury, but the type and symptoms depend on the characteristics of the injury.
- There are two main types of bladder problems after SCI:
- Spastic (reflex) bladder involves unpredictable emptying caused by overactive bladder muscles. It happens with injuries above T12.
- Flaccid (non-reflex) bladder involves an inability to empty the bladder because of “floppy” and underactive bladder muscles. It happens with injuries below T12.
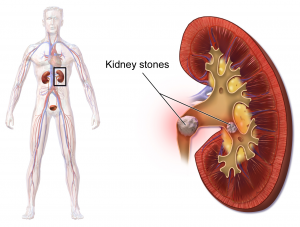
- People with SCI are also at risk of complications like urinary tract infections, autonomic dysreflexia (if above T6), kidney and bladder stones, and kidney damage.
- Bladder care after SCI involves developing a regular bladder routine that meets your unique bladder needs. This may include a variety of treatments, such as catheters, medications and injections.
The urinary system
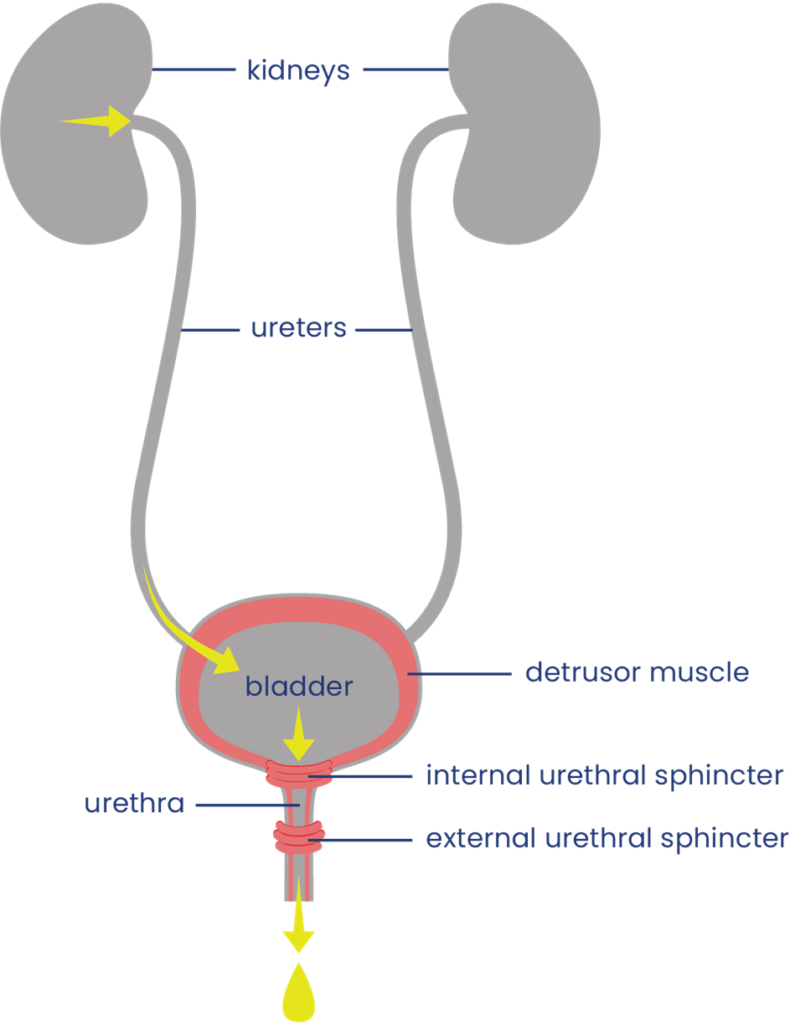 The urinary system helps the body filter and remove waste products and excess fluids. It consists of the kidneys, ureters, bladder, urethra, and bladder and sphincter muscles.
The urinary system helps the body filter and remove waste products and excess fluids. It consists of the kidneys, ureters, bladder, urethra, and bladder and sphincter muscles.
The kidneys filter the blood to produce urine, which is passed through narrow tubes called ureters to the bladder. The bladder is a sac that collects urine. Urine passes out of the body from the bladder through a tube called the urethra.
Filling and emptying of the bladder are partly controlled by the bladder muscles:
- The bladder wall muscle (detrusor muscle) is smooth muscle that covers the outside of the bladder. When it contracts, it squeezes the bladder and pushes urine out through the urethra. When it is relaxed, the bladder is loose and can be filled with urine.
- The bladder sphincter muscles (urethral sphincter or valve muscles) are two muscles which surround the exit of the bladder like a ring. When they tighten, they close off the urethra and hold urine in the bladder. When they relax, they allow urine to drain. The internal sphincter muscle is controlled unconsciously and the external sphincter muscle is controlled consciously.
Bladder function
When the bladder is not full, the bladder wall muscle is relaxed and urine produced by the kidneys passes through the ureters to fill the bladder. The bladder sphincter muscles are tightened so urine does not leak out.
When there is enough urine to stretch the bladder walls, a nerve signal is sent up the spinal cord to tell the brain that the bladder is full. Because the brain controls the external sphincter muscle, urine can be held until an appropriate time to empty.
When the bladder is to be emptied, signals are sent from the brain down the spinal cord to cause the coordinated squeezing of the bladder wall muscle and relaxation of the bladder sphincter muscles to allow urine to pass through the urethra and out of the body. Control of urination involves both bladder reflexes (in which emptying is triggered when the bladder is full) and voluntary control (in which urine can be held until a socially appropriate time to empty).
Neurogenic Bladder
Neurogenic bladder is bladder dysfunction caused by damage to the nerves, brain or spinal cord. After a spinal cord injury, nerve signals that normally allow the brain and bladder to communicate with one another cannot get through. This can affect bladder sensation and control.
Loss of bladder control
Signals from the brain are needed for the bladder muscles to contract and relax properly. If these signals cannot get through, the bladder muscles may contract too much, too little, or at the wrong times, depending on whether the person has spastic or flaccid bladder.
Reduced bladder sensation
Normally, signals that are sent up the spinal cord to the brain when the bladder is full. When the signals are interrupted, the ability to feel fullness and other sensations from the bladder may be reduced.
Bladder changes after SCI are different for everyone. Some people experience only mild changes to how the bladder works (such as greater sense of urgency when the bladder is full); while others experience complete loss of bladder sensation and control.
The symptoms of neurogenic bladder depend on the characteristics of the SCI, such as the level and completeness of the injury. There are two main types of neurogenic bladder after SCI, spastic bladder and flaccid bladder (see below).
Spastic Bladder
Spastic bladder (also called “reflex bladder” or “overactive bladder”) happens when the spinal cord is injured above T12. Spastic bladder happens because the brain can no longer control reflexes in the bladder muscles. This leads to tension in the bladder wall muscle when it is supposed to be relaxed and spasms of the bladder muscles which cause emptying.
Usually, the bladder sphincter muscles are also overactive and cannot coordinate very well with the bladder wall muscle. This is called detrusor dyssynergia or detrusor sphincter dyssynergia (DSD). When this happens, the bladder sphincter muscle tightens while the bladder wall muscle contracts, like squeezing a balloon that is tied off. This can cause high pressures within the bladder that can damage the bladder and kidneys.
Symptoms of spastic bladder:
- Loss of control of bladder emptying (incontinence), leading to random emptying (accidents), inability to empty when you want to and leaking
- Reflex emptying in response to things like touching the thigh or abdomen
- People with some bladder sensation may experience sudden strong urges or a frequent need to urinate
- Incomplete emptying of the bladder caused by poor coordination of the bladder wall muscle and bladder sphincter muscles (detrusor dyssynergia)
- Reduced or complete loss of bladder sensation
Flaccid bladder
Flaccid bladder (also called “non-reflex bladder” or “underactive bladder”) happens when the spinal cord is injured below T12-L1 (i.e cauda equina injuries). Flaccid bladder happens because there is a loss of both input from the brain and reflexes from the spinal cord. This causes the bladder wall muscle to stay loose and floppy all the time. When this happens, the bladder wall muscle cannot squeeze the bladder to empty urine.
Usually, the external sphincter muscle is also overly relaxed, causing leaking during activities like transfers and coughing. However, the internal sphincter muscle is often in spasm and does not relax enough to allow urine to pass out of the body easily.
Symptoms of flaccid bladder:
- Inability to empty the bladder, including loss of reflex emptying
- Incomplete bladder emptying, leading to some urine remaining in the bladder after emptying (urinary retention)
- Damage to the walls of the bladder when they are overstretched
- Backflow of urine back to the kidneys (reflux), which can damage the kidneys
- Reduced or complete loss of bladder sensation
Bladder examination
Bladder changes are diagnosed primarily through a bladder examination. A bladder examination typically involves several components:
- Your health provider will ask you questions about your medical history, symptoms, bladder routine, and current treatments.
- You may be asked to complete a “urinary diary” and/or detailed questionnaires about your bladder care. This often involves recording how often you empty your bladder, how much urine is produced each time, and details about your fluid intake (what you drink, when and how much).
- A physical examination may involve an inspection of the abdominal, pelvic and genital areas, as well as neurological testing of your reflexes, muscle strength, and sensation.
Other testing
Other testing may also be done if your health providers need further information.

The dipstick or urine test strip is a basic diagnostic tool for identifying presence of substances or infection in urine.3
Urine culture
A urine culture and sensitivity test involves collecting urine in a sterile container to test for infection. Urine samples are usually collected mid-stream while emptying so the test is more accurate. If the sample is collected from an indwelling catheter, the catheter should be changed first. Samples are never taken from a urine drainage bag.
Blood tests
Blood tests may be done if there is concern about kidney function, kidney damage, or an infection. This usually involves testing for blood urea nitrogen (BUN) and creatinine.
Ultrasound
Ultrasound is an imaging technique that uses sound waves to visualize deep tissues. Ultrasound imaging may be done over the kidneys (known as renal ultrasound) to detect damage, kidney stones and infections.
Urodynamic testing
Urodynamic testing includes special tests that can be used to look at bladder pressures and urine flow. It can test how the bladder acts when it fills and empties, how well it coordinates, and the pressure within the bladder. This test may involve urinating into a special container that can measure the flow and volume of urine, insertion of a catheter to measure the leftover urine, and inserting water into the bladder to measure your ability to prevent emptying. It may also involve placing a small catheter into the rectum that measures the electrical activity of muscles.
Typical urodynamic measures
Bladder Capacity: The amount of urine the bladder can hold.
Voiding Efficiency: The amount of urine voided compared to the amount in the bladder before voiding. More efficiency means less urine is left in the bladder.
Bladder Compliance: The ability of the bladder to stretch in response to an increased amount of urine in the bladder. Without enough stretch there will be large increases in pressure, which is damaging to the urinary tract.
Imaging
Other imaging, such as x-ray, computed tomography (CT), and magnetic resonance imaging (MRI) are sometimes used for further investigation of bladder problems.
Cystoscopy
Cystoscopy (sometimes known as a “bladder scope”) is the use of a very small camera that can be inserted into the urethra to look at the urinary tract. Cystoscopy can be used to identify bladder stones, bladder health issues or damage including bladder cancer. It can also perform therapeutic procedures if needed such as removing tissue or stones.
Early bladder care
In the early hospital phase right after injury, the circulatory system is stabilizing, and the prevention of infections and other complications is the priority. During this phase, an indwelling catheter is placed in the bladder to constantly drain urine from the bladder. The catheter will be changed regularly and maintained in a sterile way by your nurse.
After the acute phase, bladder care will involve transitioning to more long-term bladder care techniques and developing a suitable bladder routine.
Bladder Routine
A bladder routine is a regular routine of bladder techniques and treatments that are done every day to maintain bladder function and health. This usually involves techniques to regularly empty the bladder, prevent leaks, and avoid serious complications long-term.
Every person’s routine is different and often involves trial and error to find the methods that best meet your unique symptoms, abilities, preferences, and lifestyle. There is a wide range of different techniques and treatments that may make up your routine, including catheters, medications, and methods of stimulation like electrical stimulation. Keep in mind that spastic bladder and flaccid bladder happen for different reasons and are managed differently.
Other things to consider when developing a bladder routine:
- Timing and amount of fluids
- Caffeine and alcohol consumption
- Scheduling of bladder emptying (such as how long between catheterizations, before going to bed or certain activities, after drinking fluids)
- What type of equipment to use, such as type of catheter and collection bag for different situations
- What to do if you have a bladder infection or other new health problem
- Regular assessment of bladder care with your health team
Spastic bladder management
The goals of spastic bladder management are to reduce overactivity in the bladder wall muscle which causes accidents, leaking, and wetness; as well as preventing high pressures within the bladder. This may include treatments such as:
- Indwelling catheters, condom catheters, and/or intermittent catheterization to drain the bladder
- Reflex voiding may help to empty the bladder for some people
- Anticholinergic medications may help to relax the bladder muscles
- Botulinum toxin (Botox) injections to help relax the bladder muscles
- Bladder augmentation surgery to increase the capacity of the bladder to hold urine
Flaccid bladder management
The goals of flaccid bladder management are to regularly empty the bladder to prevent overfilling and increased pressure in the bladder; and to prevent leaking and wetness. This may include treatments such as:
- Intermittent catheterization or indwelling catheters
- Condom catheters or pouches may be used to catch leaks but not for emptying
- Alpha-adrenergic blockers may help to relax the bladder sphincter muscles
- Botulinum toxin (Botox) injections
- Surgical techniques such as sphincterotomy or stents
Urinary catheters are pieces of equipment that are used to drain urine from the bladder. There are many different ways that catheters are used.
Intermittent catheterization
Intermittent catheterization is when a catheter is inserted and removed through the urethra to drain the bladder at regular intervals throughout the day. Bladder emptying with intermittent catheterization must be done hygienically and on a regular schedule.
Intermittent catheterization is usually used by people who have enough hand function to perform the procedure independently. It is the closest method to normal bladder function, where the bladder fills continuously for a period of time and then empties all at once.
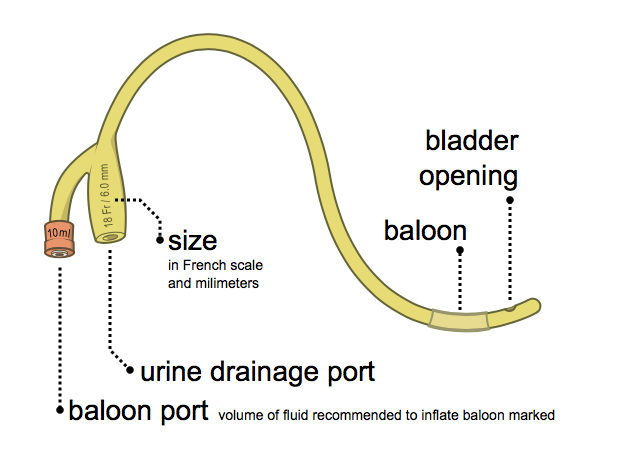
Indwelling catheters
Indwelling catheters (such as Foley catheters) are catheters that are inserted directly into the bladder and remain in place to continually drain the bladder. Indwelling catheters may be inserted through the urethra (urethral catheters) or through a surgically created hole through the abdomen (suprapubic catheters).
Indwelling catheters are usually used if inserting your own catheter independently is difficult or there are concerns about leaking between sessions of emptying.
Condom catheters (only for males)
Condom catheters are catheters that resemble a condom and are placed over the penis and connected through tubes to a collection device. Condom catheters are usually used by people that leak in between emptying or for individuals who have the ability to trigger emptying by causing a spasm of their bladder (reflex voiding).
One of the main concerns of condom catheters is incomplete bladder drainage, which can cause kidney damage. A careful medical examination is needed to ensure that condom catheters are a safe option for use.
Refer to our article on Urinary Catheters for more information!
Catching leaks
Some people may use medical “penis pouches” (loosely fitted bags that can be placed around the penis), pads, or other devices to catch small leaks in between catheterizations. These will depend on the person and their risk of other problems like pressure injuries, and should be discussed in detail with your health providers before use.
Reflex voiding is a technique that can be used by some people with spastic bladder to stimulate urination. Reflex voiding is usually done by tapping over the bladder lightly and repeatedly with the fingertips or the side of the hand to stimulate reflexes in the bladder muscles. This technique can be used to help improve bladder emptying during intermittent catheterization and when using condom catheters. However, only a small number of people can use this technique safely without increasing the pressure too high in the bladder. Speak to your health team for more information about this technique.
Many reflex voiding techniques are not recommended
Older techniques for reflex voiding such as the Valsalva maneuver (increasing abdominal pressure by holding the breath and bracing) and the Credé technique (applying manual pressure onto the bladder through the abdomen) are no longer recommended because they can cause too much pressure in the bladder, which can damage the kidneys.
Several medications may be used to help manage bladder problems after SCI. These may help to relax overactive muscles or cause the bladder muscles to contract, depending on the type of bladder change experienced. A number of other medications may also be used for different aspects of bladder treatment after SCI.
Inserting liquid medications into the bladder
Some medications may be dissolved in a liquid solution and introduced into the bladder through a catheter after emptying. The solution is then left in the bladder until the next urination. This is called an intravesical instillation. Intravesical instillations may be used because their effects are more specific to the bladder, instead of throughout the whole body as with oral medications.
Anticholinergic medications
 Anticholinergic medications (sometimes called antimuscarinic medications) are used to relax muscle spasms in the bladder wall muscle. This can help to reduce pressure within the bladder, increase the ability of the bladder to hold urine, and help reduce incontinence.
Anticholinergic medications (sometimes called antimuscarinic medications) are used to relax muscle spasms in the bladder wall muscle. This can help to reduce pressure within the bladder, increase the ability of the bladder to hold urine, and help reduce incontinence.
There are many different types of anticholinergic medications, with the most common being:
- oxybutynin (Ditropan, Ditropal XL, Oxytrol, Uromax)
- tolterodine (Detrol)
- fesoterodine (Toviaz)
- trospium chloride (TCL, Trosec)
- propiverine hydrochloride (Mictonorm)
- darifenacin (Enablex)
- solifenacin (Vesicare)
These can be taken by mouth or administered directly into the bladder in a liquid form.
Alpha-adrenergic blockers
Alpha-adrenergic blockers are medications that are used to encourage the bladder sphincter muscles to relax to allow urine to flow out of the body. This can help with bladder emptying and help prevent urinary retention. Common alpha-adrenergic blockers that may be used include tamulosin, mosixylyte, terazosin, and phenoxybenzamine.
Botulinum toxin injections
 Injecting small doses of some strains of botulinum toxin (Botox) into muscles can help to reduce muscle spasms. Injections into the bladder wall muscle or the external sphincter muscle can help to relax these muscles to help prevent leaking and incontinence or to improve bladder emptying. The effects of these injections can last for 6 to 12 months.
Injecting small doses of some strains of botulinum toxin (Botox) into muscles can help to reduce muscle spasms. Injections into the bladder wall muscle or the external sphincter muscle can help to relax these muscles to help prevent leaking and incontinence or to improve bladder emptying. The effects of these injections can last for 6 to 12 months.
Read our article on Botulinum Toxin for more information!
Other medications
Capsaicin, a chemical commonly found in hot peppers, and its derivative resiniferatoxin, may be administered as a liquid into the bladder to help reduce urinary frequency, leaking, and bladder pressures related to bladder wall muscle overactivity, and increase bladder capacity.
- Nociceptin/orphanin phenylalanine glutamine is another medication with effects similar to capsaicin and resiniferatoxin. It may also be given into the bladder to reduce overactivity in the bladder wall muscle.
- Medications that are normally used to treat spasticity may also help with bladder problems related to spastic bladder. For example, baclofen and clonidine may help with bladder function after SCI.
- Phosphodiesterase-5 (PDE5) inhibitors such as tadalafil and vardenafil may help to reduce overactivity in the bladder wall muscle and increase bladder capacity.
- 4-Aminopyridine (fampridine) improves the transfer of nerve signals, which may help individuals regain sensation and control of the bladder sphincter muscles to improve emptying.
Bladder surgery is usually only considered if other less-invasive treatments are not effective. Surgical procedures that may be used include the Mitrofanoff procedure, bladder augmentation, sphincterotomy (for males), and urethral stents.
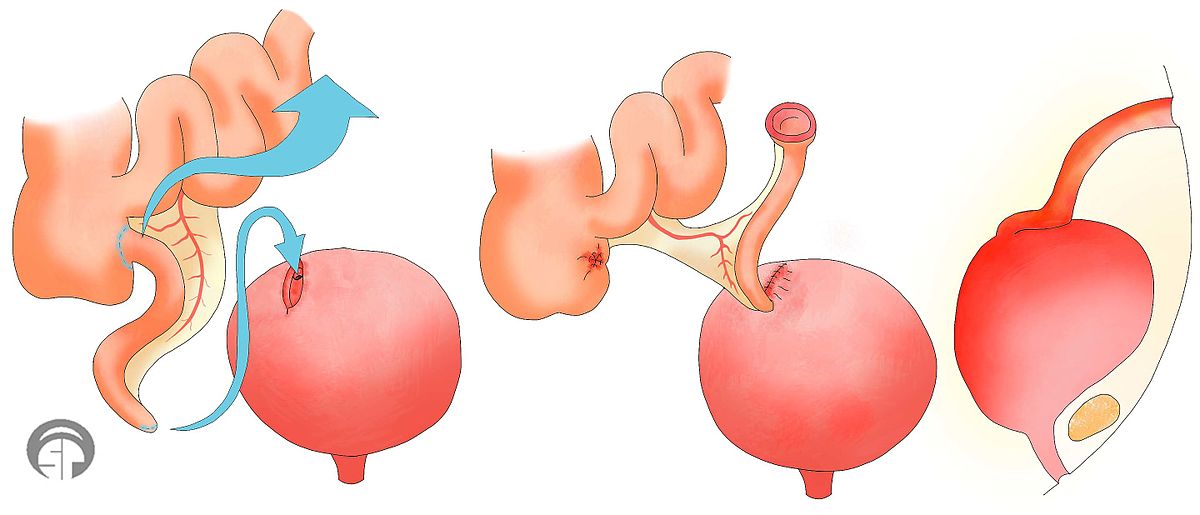
Mitrofanoff procedure
The Mitrofanoff procedure involves the use of the appendix or part of the intestine to create a channel between the abdomen and bladder. The channel self-seals shut when the catheter is removed. This channel can be used for insertion of a catheter for intermittent catheterization. The urine can then be drained into a cup or toilet. This may be useful for people who have difficulty self-catheterizing directly into the urethra and is often used for women (who have greater difficulty inserting catheters).
Bladder augmentation
Bladder augmentation (also called augmentation cystoplasty) is a procedure in which the bladder is made bigger to create more room to hold urine. This is done by removing a segment of the intestine and stitching this tissue to an incision into the bladder to make the bladder bigger. Bladder augmentation may help to reduce pressure in the bladder and help to prevent incontinence related to spastic bladder.
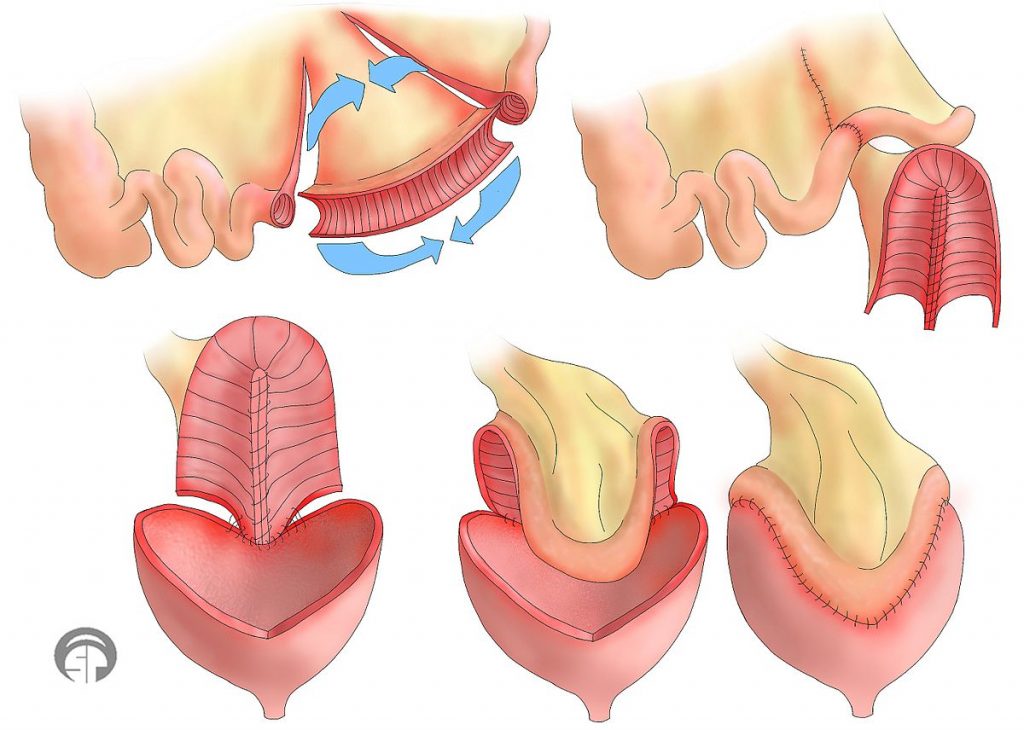
Bladder augmentation is a surgical procedure done to enlarge the bladder by using parts of the intestine.10
Sphincterotomy (for males)
Sphincterotomy is a surgical procedure where the internal sphincter muscle (the circular muscle that surrounds the outlet of the bladder) is cut to weaken the muscle. This is done to improve bladder emptying if this muscle is causing difficulties emptying. After a sphincterotomy, bladder emptying will happen; therefore, you must wear a collection device.
Urethral stents
Urethral stents are prosthetic tubes (usually coils of metal) with openings on both sides that are be inserted into the opening of the bladder to hold it open. This is done to allow for improved bladder emptying for people with difficulty emptying due to overactivity in the bladder sphincter muscles.
Electrical stimulation
Electrical stimulation can be used to help normalize the activity of the bladder muscles and control of bladder emptying. This may involve the implantation of a stimulator and electrodes that stimulate the sacral nerves that send brain signals to the bladder. This is sometimes referred to as neuromodulation.
Commercially available electrical bladder stimulation systems may be used for this purpose. However, these systems may be expensive and is not available in all locations.
Refer to our articles on Neuromodulation for more information!
Acupuncture
Acupuncture and electroacupuncture have also been suggested as treatment options to help with bladder function by influencing nerve signals related to bladder function.

Watch SCIRE’s video on bladder care to understand more about managing your bladder as you age.11
Typically, people will experience changes in urination as they age. Some may need to go to the bathroom more often because the bladder can’t store as much urine, or find that the flow of urine is weaker. Others may experience more urine leaking because the muscles that keep the bladder closed are weaker or the bladder contracts when it is not supposed to. The kidneys that filter your blood and produce urine may not function as well, and the risk of developing kidney stones increases.
Women, both during and post-menopause may experience an increase in the frequency of UTIs.
The aging bladder in SCI
For people aging with SCI, the long-term use of indwelling catheters to manage the bladder increases risk for bladder stones, bladder cancer, UTIs, and urinary tract deterioration. Long-term use of intermittent catheterization can increase risk for urethral strictures. People with neurogenic bladder can experience high pressures in the urinary tract and urine back up to the kidneys. Over time, this damages the structures of the urinary system and increases the risk for stones.
Bladder changes that people aging with SCI may experience: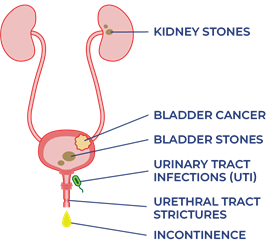
- Increased UTIs
- Increased urine leaking (incontinence)
- Urethral strictures
- Bladder/kidney stones
- Increased risk of bladder cancer
- Damage to urinary tracts and kidneys
- Reduced kidney function
Bladder changes may also trigger spasticity and autonomic dysreflexia. Other aspects of aging like pain, osteoarthritis, decreased strength and mobility, and thinning of the skin, may also affect the ability to carry out bladder routines.
All of the changes from aging listed above could lead to a need to reassess bladder management strategies.
Refer to the “What other complications are related to bladder changes?” section for more information on specific bladder problems.
Managing bladder changes with age
If you experience changes to your bladder function as you age with SCI, consult regularly with a health care provider about your bladder management. Your family doctor, physiatrist, or urologist may be familiar with the check-ins necessary for bladder care as you age with a SCI.
Strategies to consider for the management of bladder changes may include:
- Changing the catheterization method to reduce UTIs, avoid urinary tract damage, or accommodate reduced hand and wrist function
- Screenings for bladder/kidney stones and urinary tract/kidney damage
- Regular screening for bladder cancer if indwelling catheter has been used for 5-10 years
- Consulting with professionals to determine the root cause of recurrent UTIs
- Antibiotic medication to treat UTIs (only use if experiencing symptoms to avoid developing antibiotic resistance)
- Supplements to prevent UTIs (i.e. D-mannose)
- Botulinum toxin (Botox) injections to relax bladder muscles
- Surgical procedures to make the bladder bigger, improve emptying, or make catheterization easier
- Additional caregiver help
- Quit or avoid smoking (smoking can up to quadruple risk for bladder cancer)
Many people with SCI change their bladder management as they age. In one study, half of participants changed their bladder management methods over 20 years. Over time, men who used condom drainage and women who used straining to manage their bladder were most likely to change their management methods. The use of indwelling catheters increased for men. A change to intermittent catheters or suprapubic indwelling catheters for bladder management increased for both men and women.
There is research looking at the potential for epidural and transcutaneous nerve stimulation to improve bladder function and management. This may be available depending on your location.
When do I need to review or change my bladder routine?
- Your current routine is not working anymore.
- The time and energy you spend on bladder management is limiting time spent with family/friends or doing things you love.
Questions to ask yourself to manage bladder changes
- What has changed (issues, challenges, barriers)?
- When did the change start?
- What is the root cause of the change? (medical illness, injury, surgery, mobility, weakness, cognitive changes, social changes, financial changes)
- What treatments or recommendations have been tried so far?
- What has worked and what has not?
- When was the most recent review of bladder with a family doctor or specialist such as a urologist or physiatrist?
Refer to the “Medications and injections”, “Bladder surgery and stents”, and “What other complications are related to bladder changes?” sections above for more information.)
Bladder changes are common after SCI. Bladder care is an important part of self-management after SCI to prevent complications and maintain good health and quality of life.
Bladder care after SCI involves developing a regular bladder routine that meets your unique bladder needs. This may include a variety of techniques and treatments, such as catheters, medications, injections and other treatments. Speak to your health team about which bladder management options are best for you. Regular follow up with your doctor is recommended yearly.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
This page has been adapted from the SCIRE Professional “Bladder Management” Module:
Hsieh J, McIntyre A, Iruthayarajah J, Loh E, Ethans K, Mehta S, Wolfe D, Teasell R. (2014). Bladder Management Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0: p 1-196.
Available from: scireproject.com/evidence/bladder-management/
Biering-Sorensen F. Urinary tract infection in individuals with spinal cord lesion. Curr Opin Urol 2002;12(1):45-49.
Chancellor MB, Bennett C, Simoneau AR, Finocchiaro MV, Kline C, Bennett JK et al. Sphincteric stent versus external sphincterotomy in spinal cord injured men: Prospective randomized multicenter trial. J Urol 1999;161(6):1893-1898.
Chancellor MB, Karasick S, Strup S, Abdill CK, Hirsch IH, Staas WE. Transurethral balloon dilation of the external urinary sphincter: Effectiveness in spinal cord-injured men with detrusor-external urethral sphincter dyssynergia. Radiology 1993b;187(2):557-560.
Chancellor MB, Karusick S, Erhard MJ, Abdill CK, Liu JB, Goldberg BB, Staas WE. Placement of a wire mesh prosthesis in the external urinary sphincter of men with spinal cord injuries. Radiology 1993c;187(2):551-555.
Chartier-Kastler E, Amarenco G, Lindbo L, Soljanik I, Andersen HL, Bagi P, Gjodsbol K, Domurath B. A prospective, randomized, crossover, multicenter study comparing quality of life using compact versus standard catheter for intermittent self-catheterization. J Urol 2013;190:942-947.
Cheng P-T, Wong M-K, Chang P-L. A therapeutic trial of acupuncture in neurogenic bladder of spinal cord injured patients-A preliminary report. Spinal Cord 1998;36(7):476-480.
Creasey GH, Grill JH, Korsten M, HS U, Betz R, Anderson R et al. An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: A multicenter trial. Arch Phys Med Rehabil 2001;82(11):1512-1519.
Das A, Chancellor MB, Watanabe T, Sedor J, Rivas DA. Intravesical capsaicin in neurologic impaired patients with detrusor hyperreflexia. J Spinal Cord Med 1996;19(3):190-193.
DeSeze M, Wiart L, de Seze MP, Soyeur L, Dosque JP, Blajezewski S et al. Intravesical capsaicin versus resiniferatoxin for the treatment of detrusor hyperreflexia in spinal cord injured patients: A double-blind, randomized, controlled study. J Urol 2004;171(1):251-255.
DeSeze M, Wiart L, Joseph PA, Dosque JP, Mazaux JM, Barat M. Capsaicin and neurogenic detrusor hyperreflexia: A double-blind placebo-controlled study in 20 patients with spinal cord lesions. Neurourol Urodyn 1998;17(5):513-523.
DeVivo, M.J. Sir Ludwig Guttman Lecture: Trends in SCI rehabilitation outcomes from model systems in the United States:1973-2006. Spinal Cord 2007;45(11):713-721.
Evans RJ. Intravesical therapy for overactive bladder. Current Urology Reports 2005;6:429-433.
Farag FF, Martens FM, Rijkhoff NJ, Heesakkers JP. Dorsal genital nerve stimulation in patients with detrusor overactivity: A systematic review. Curr Urol Rep 2012;12(5):385-388.
Foxman B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Dis Mon;49(2):53-70.
Gobeaux N, Yates DR, Denys P, Even‐Schneider A, Richard F, Chartier‐Kastler E. Supratrigonal cystectomy with hautmann pouch as treatment for neurogenic bladder in spinal cord injury patients: Long‐term functional results. Neurourology and urodynamics 2012;31(5):672-676.
Goldman HB, Amundsen CL, Mangel J, Grill J, Bennet M, Gustafson KJ, Grill WM. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol Urodyn 2008;27(6):499-503.
Greenstein A, Rucker KS, Katz PG. Voiding by increased abdominal pressure in male spinal cord injury patients–long term follow up. Paraplegia 1992;30(4):253-255.
Grijalva I, Garcia-Perez A, Diaz J, Aguilar S, Mino D, Santiago-Rodriguez E, et al. High doses of 4-aminopyridine improve functionality in chronic complete spinal cord injury patients with MRI evidence of cord continuity. Arch Med Res 2010;41:567-575.
Groah SL, Weitzenkamp DA, Lammertse DP, Whiteneck GG, Lezotte DC, Hamman RF. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil 2002;83(3):346-351.
Groah SL, Weitzenkamp DA, Lammertse DP, Whiteneck GG, Lezotte DC, Hamman RF. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil 2002;83(3):346-351.
Gurung PM, Attar KH, Abdul-Rahman A, Morris T, Hamid R, Shah PJ. Long-term outcomes of augmentation ileocystoplasty inpatients with spinal cord injury: A minimum of 10 years of follow-up. BJU Int 2012;109(8):1236-1242.
Hackler RH. Long-term Suprapubic cystostomy drainage in spinal cord injury patients. Br J Urol 1982;54(2):120-121.
Hakenberg OW, Ebermayer J, Manseck A, Wirth MP. Application of the Mitrofanoff principle for intermittent self-catheterization in quadriplegic patients. Urology 2001;58(1):38-42.
Hansen J, Media S, Nohr M, Biering-Sorensen F, Sinkjaer T, Rijkhoff NJ. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol 2005;173(6):2035-2039.
Hassouna M, Elmayergi N, Abdelhady M. Update on sacral neuromodulation: Indications and outcomes. Curr Urol Rep 2003 Oct;4(5):391-398.
Hikita K, Honda M, Kawamoto B, Panagiota T, Inoue S, Hinata N. Botulinum toxin type A injection for neurogenic detrusor overactivity: Clinical outcome in Japanese patients. International J Urol 2013;20(1):94-99.
Hohenfellner M, Humke J, Hampel C, Dahms S, Matzel K, Roth S, Thuroff JW, Schultz-Lampel D. Chronic sacral neuromodulation for treatment of neurogenic bladder dysfunction: Long-term results with unilateral implants. Urology 2001;58(6):887-892.
Horvath EE, Yoo PB, Amundsen CL, Webster GD, Grill WM. Conditional and continuous electrical stiulation increase cystometric capacity in persons with spinal cord injury. Neurourol Urodyn 2010;29(3): 401-407.
Juma S, Mostafavi M, Joseph A. Sphincterotomy: Long-term complications and warning signs. Neurourol Urodyn 1995;14(1):33-41
Katsumi HK, Kalisvaart JF, Ronningen LD, Hovey Rm. Urethral versus suprapubic catheter: Choosing the best bladder management for male spinal cord injury patients with indwelling catheters. Spinal Cord 2010;48(4):325-329.
Katz PG, Greenstein A, Severs SL, Zampieri TA, Singh SK. Effect of implanted epidural stimulator on lower urinary tract function in spinal-cord-injured patients. Eur Urol 1991;20(2):103-106.
Kaufman JM, Fam B, Jacobs SC, Gabilondo F, Yalla S, Kane JP et al. Bladder cancer and squamous metaplasia in spinal cord injury patients. J Urol 1977;118(6):967-971.
Kim JH, Rivas DA, Shenot PJ, Green B, Kennelly M, Erickson JR et al. Intravesical resiniferatoxin for refractory detrusor hyperreflexia: A multicenter, blinded, randomized, placebo-controlled trial. J Spinal Cord Med 2003;26(4):358-363.
Kirkham AP, Knight SL, Craggs MD, Casey AT, Shah PJ. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord 2002;40(6):272-281.
Kirkham APS, Shah NC, Knight SL, Shah PJR, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord 2001;39(8):420-428.
Kutzenberger J, Domurath B, Sauerwein D. Spastic bladder and spinal cord injury: Seventeen years of experience with sacral deafferentation and implantation of an anterior root stimulator. Artif Organs 2005;29(3):239-241.
Lazzeri M, Calo G, Spinelli M, Guerrini R, Salvadori S, Beneforti P et al. Urodynamic effects of intravesical nociceptin/orphanin FQ in neurogenic detrusor overactivity: A randomized, placebo-controlled, double-blind study. Urology 2003;61(5):946-950.
Lazzeri M, Spinelli M, Beneforti, P, Zanollo A, Turini D. Intravesical resiniferatoxin for the treatment of detrusor hyperreflexia refractory to capsaicin in patients with chronic spinal cord diseases. Scandinavian J Urol and nephrology 1998;32(5):331-334.
Locke JR, Hill DE, Walzer Y. Incidence of squamous cell carcinoma in patients with long-term catheter drainage. J Urol 1985;133(6):1034-1035.
Lombardi G, Del PG. Clinical outcome of sacral neuromodulation in incomplete spinal cord injured patients suffering from neurogenic lower urinary tract symptoms. Spinal Cord 2009;47:486-491.
Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40(5):643-654.
Ord J, Lunn D, Reynard J. Bladder management and risk of bladder stone formation in spinal cord injured patients. Journal d’urologie 2003;170(5):1734-1737.
Pan D, Troy A, Rogerson J, Bolton D, Brown D, Lawrentschuk N. Long-term outcomes of external sphincterotomy in a spinal injured population. J Urol 2009;181:705-709.
Perkash I, Kabalin JN, Lennon S, Wolfe V. Use of penile prostheses to maintain external condom catheter drainage in spinal cord injury patients. Paraplegia 1992;30(5):327-332.
Perkash I. Efficacy and safety of terazosin to improve voiding in spinal cord injury patients. J Spinal Cord Med 1995;18(4):236-239.
Petersen T, Nielsen J, Schrøder H. Intravesical capsaicin in patients with detrusor hyper-reflexia: A placebo-controlled cross-over study. Scandinavian J Urol and nephrology 1999;33(2):104-110.
Popovic MR. Sacral root stimulation. Spinal Cord. 2002 Sep;40(9):431.
Sanford MT, Suskind AM. Neuromodulation in neurogenic bladder. Transl Androl Urol. 2016 Feb;5(1):117-26.
Sheriff MK, Foley S, McFarlane J, Nauth-Misir R, Craggs M, Shah PJ. Long-term suprapubic catheterisation: Clinical outcome and satisfaction survey. Spinal Cord 1998;36(3):171-176.
Sievert KD, Amend B, Gakis G, Toomey P, Badke A, Kaps HP, Stenzl A. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol 2010;67:74-84.
Woodbury MG, Hayes KC, Askes HK. Intermittent catheterization practices following spinal cord injury: A national survey. Can J Urol 2008;15(3):4065-4071.
Wyndaele JJ, Madersbacher H, Kovindha A. Conservative treatment of the neuropathic bladder in spinal cord injured patients. Spinal Cord 2001;39(6):294-300.
Wyndaele JJ. Conservative treatment of patients with neurogenic bladder. European Urology Supplements 2008;7(8):557-565.
Evidence for “How does aging affect the bladder with SCI?” is based on:
Charlifue S, Jha A, Lammertse D. Aging with Spinal Cord Injury. Phys Med Rehabil Clin N Am. 2010;21(2):383-402. doi:10.1016/j.pmr.2009.12.002
Raz R. Hormone Replacement Therapy or Prophylaxis in Postmenopausal Women with Recurrent Urinary Tract Infection. J Infect Dis. 2001;183(s1):S74-S76. doi:10.1086/318842
West DA, Cummings JM, Longo WE, Virgo KS, Johnson FE, Parra RO. Role of chronic catheterization in the development of bladder cancer in patients with spinal cord injury. Urology. 1999;53(2):292-297. doi:10.1016/S0090-4295(98)00517-2
Zhang Z, Liao L. Risk factors predicting upper urinary tract deterioration in patients with spinal cord injury: a prospective study. Spinal Cord. 2014;52(6):468-471. doi:10.1038/sc.2014.63
Pavlicek D, Krebs J, Capossela S, et al. Immunosenescence in persons with spinal cord injury in relation to urinary tract infections -a cross-sectional study-. Immunity & Ageing. 2017;14(1):22. doi:10.1186/s12979-017-0103-6
Image Credits:
- The Urinary System ©SCIRE, CC BY-NC 4.0
- Modified from: Nephron Anatomy ©BruceBlaus, CC BY-SA 4.0
- Dipstick ©SCIRE, CC BY-NC 4.0
- Foley catheter EN ©Ikej Renesz, CC BY-SA 3.0
- Cewnik zewnetrzny 0211 ©Sobol2222 assumed (based on copyright claims), CC0 1.0
- Medications ©Steve Buissinne, CC0 1.0
- Syringe ©Arek Socha, CC0 1.0
- Chili ©PublicDomainPictures, CC0 1.0
- Mitrofanoff ©Aphelpsmd, CC BY-SA 4.0
- Ileocystoplasty JPEG ©Aphelpsmd, CC BY-SA 4.0
- Aging Bladder Thumbnail ©SCIRE, CC BY-NC 4.0
- Urinary System Aging Changes ©SCIRE, CC BY-NC 4.0
Disclaimer: This document does not provide medical advice. This information is provided for educational purposes only. Consult a qualified health professional for further information or specific medical advice. The SCIRE Project, its partners and collaborators disclaim any liability to any party for any loss or damage by errors or omissions in this publication.
Author: SCIRE Community Team | Reviewer: Darryl Caves | Published: 17 January 2018 | Updated: ~
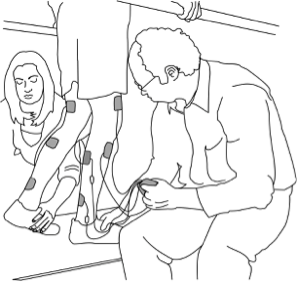
Standing with supportive equipment is a therapy option after spinal cord injury (SCI). This page outlines basic information about the use of supported standing after SCI.
Key Points
- Passive standing using supportive equipment is a therapy option for people who do not stand as part of their everyday mobility.
- Passive standing involves using equipment such as standing frames, tilt tables, orthoses or standing wheelchairs to support an upright position for a period of time.
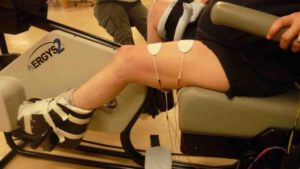
- Standing involves a change in posture that challenges the circulatory system, loads the legs, and provides different sensory stimulation.
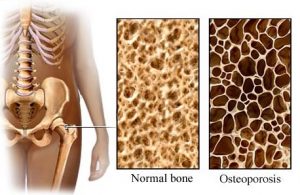
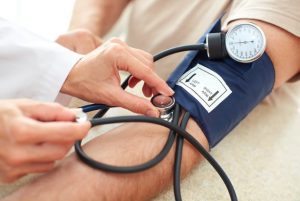
- Research evidence suggests that standing may improve blood pressure control and spasticity management. There is conflicting evidence about whether passive standing helps with bone density or bowel and bladder health.
- Further research is needed to better understand the benefits of standing after SCI and how long standing should be done for to achieve those benefits.
Standing is an important part of functional movement in humans. Standing is needed for walking, and also provides a challenge to the circulatory system, bones, and muscles in ways that cannot be achieved in sitting or lying.
Passive standing (standing with support instead of by muscle activation) may have treatment benefits after SCI, even when the recovery of walking and standing abilities is unlikely. Standing may have benefits in treating health conditions associated with SCI, such as conditions involving the musculoskeletal, circulatory, breathing, bowel and bladder systems. It remains a key treatment tool used in rehabilitation.
Supported standing involves the use of special equipment to support an upright posture. The type of equipment used depends on the person’s unique characteristics and abilities (such as the amount of muscle control in the arms, legs and trunk), the equipment that is available, and other medical concerns like joint contractures, spasticity and osteoporosis. Equipment used for standing may include a wide range of different devices such as:
Tilt tables
Tilt tables are flat surfaces that can be tilted from a horizontal position into a vertical standing position. The person is strapped securely to the table while in a horizontal lying position and the table can be tilted vertically. Tilt tables are typically the first devices that are used to work towards standing because the table can be gradually increased by degrees. This is often needed because it may take some time to tolerate being upright and maintaining a safe blood pressure. It is also a good device to test a person’s physical tolerance and safety for standing.
Standing frames
Standing frames are simple frames that have padding at the joints to support a standing position. There are many different types of standing frames. The frame needs to be fitted to the person’s unique physical abilities and body type and minimize areas of excess pressure.
Standing wheelchairs
Standing wheelchairs are wheelchairs that can extend from a sitting position into standing. There are many different types of standing wheelchairs, from manual devices to motorized systems. However, standing wheelchairs are expensive and not commonly available.
Body weight-supported treadmill training
Suspension body weight support systems involve a harness system that is suspended from above to support a percentage of body weight while standing. These systems are typically used while walking on a treadmill (body weight-supported treadmill training) or sometimes while walking over ground. This type of system is usually used for people with incomplete injuries that may work towards standing or stepping independently.
See our article on Body Weight Supported Treadmill Training.
Robotic exoskeletons
Robotic exoskeletons are a relatively new and emerging technology that is typically used for walking and walking training, but may also have benefits related to standing. However, this equipment is costly and not available in most settings.
Walkers, crutches or canes may be used by people with incomplete SCI and good strength in the arms who need only minimal support in standing.
Orthoses
Orthoses and braces may be used to brace the hip, knee and/or ankle joints to keep them from bending. This can help to support the person in an upright standing posture with training and rehabilitation. Orthoses and braces are typically custom-made and usually used by people with paraplegia who have good upper body strength and hip flexibility. Orthoses and braces used for standing may include:
- Knee Ankle Foot Orthoses (KAFOs) provide support at the knee, ankle and foot.
- Reciprocating Gait Orthoses (RGOs) are more complex orthoses that are made of a left and right KAFO that are linked together with a rigid brace at the pelvis or abdomen. The brace has hip joints that are built with an alternating stepping mechanism. When one leg is extended, the other flexes forward, providing assistance for stepping. Although normally used for walking, reciprocating gait orthoses can also be used to help support a standing position.
Functional electrical stimulation
Functional electrical stimulation (FES) involves the use of electrical stimulation to activate muscles that are weak or paralyzed after an SCI during a purposeful activity. FES over the trunk or leg muscles may be used while standing with equipment for added benefits.
See our article on Functional Electrical Stimulation (FES) for more information.
Standing equipment may be expensive and sometimes requires repeated visits to healthcare facilities, which can sometimes be a barrier to regular standing. It is important for the individual to work with their health providers to find appropriate equipment that is safe and suitable.
Once appropriate equipment and strategies for standing are selected with assistance from a health provider, standing is gradually introduced over time. The amount of time spent standing, the amount of load that is taken through the legs, and the final standing position will be adjusted until a suitable standing position can be maintained. During these first several sessions, health providers will monitor for any adverse effects related to the treatment.
Current research findings are unable to tell us how long or how often standing should be done to have benefits. Studies have used standing for 20 to 60 minutes, three to four times per week to study the effects of this treatment. It will be different for everyone. The standing prescription will be based on the person’s unique situation.
Depending on the treatment goals, standing may also involve:
- Adding extra weight while standing
- Using standing together with functional electrical stimulation (FES) to activate the muscles of the legs and/or trunk
- Weight-shifting, balance or stepping activities
Supported standing is considered to be a relatively safe treatment for use after SCI. However, there are some situations in which standing may not be appropriate and some possible risks. This is not a complete list; please consult a health provider for further safety information.
Standing should not be used in the following situations:
- By people with recent broken bones (fractures) or a high risk of fractures (such as people with severe osteoporosis)
- Where the standing equipment places excess pressure on areas where there are injuries, sores, and wounds; or areas of skin prone to pressure injuries
- By individuals whose limbs cannot be brought into a good standing position due to other conditions like joint contractures, spasticity, or heterotopic ossification
- By people with medical conditions where heart rate or blood pressure are uncontrolled, such as those who are unable to stay upright without a major drop in blood pressure (severe orthostatic hypotension)
- By people with muscle or joint injuries or other conditions that may be worsened by standing
Risks of standing may include:
- Pressure injuries if the position and equipment used for standing creates too much pressure or shear while standing – it is essential that the equipment used for standing is appropriately fitted to prevent skin damage
- Blood pooling in the legs may lead to feelings of light-headedness, dizziness or fainting (orthostatic hypotension)
- Broken bones (fractures) are possible in weight-bearing positions in people with osteoporosis
- Increased spasticity or autonomic dysreflexia in some people
- Pain in the standing position
For more information on these topics, see our articles on Pressure Injuries, Orthrostatic Hypotension, Osteoporosis, Spasticity, and Autonomic Dysreflexia
If using electrical stimulation, the safety precautions and risks associated with use of functional electrical stimulation (FES) also apply.
Bone health
It is not clear if standing helps to maintain or increase bone density in the legs after SCI. Current research evidence is inconclusive and further studies are needed.
Blood pressure and circulation
Another proposed use of standing after SCI is to help with blood pressure control. One study provides weak evidence that standing with a harness and assistance from health providers helps to increase resting blood pressure and reduce drops in blood pressure when standing (orthostatic hypotension) in people with cervical SCI.
Spasticity
There is weak evidence that standing may help to reduce spasticity short term in people with SCI. There are also surveys that report that many people with SCI report that regular standing helped to reduce their spasticity.
Bowel problems
There is not enough evidence to determine whether standing can also improve bowel function. Further research in this area is needed.
Supported standing serves as a therapy option for people such as individuals with SCI as they do not normally stand as part of their everyday mobility. This therapy involves equipment such as standing frames, tilt tables, orthoses, or standing wheelchairs to support an upright position for a designated period of time. While there is research supporting the benefits of supported standing, conflicting evidence is still apparent. Further research is needed to better understand the benefits of supported standing after SCI.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Professional “Bone Health”, “Orthostatic Hypotension”, “Spasticity”, and “Bowel Dysfunction and Management” Modules:
Craven C, Lynch CL, Eng JJ (2014). Bone Health Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 37.
Available from: https://scireproject.com/evidence/bone-health/
Krassioukov A, Wecht JM, Teasell RW, Eng JJ (2014). Orthostatic Hypotension Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p. 1-26.
Available from: https://scireproject.com/evidence/orthostatic-hypotension/
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Curt A, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0.
Available from: https://scireproject.com/evidence/spasticity/
Coggrave M, Mills P, Willms R, Eng JJ, (2014). Bowel Dysfunction and Management Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1- 48.
Available from: https://scireproject.com/evidence/bowel-dysfunction-and-management/
Evidence for “Does standing work for treating the symptoms of SCI?” is based on the following studies:
Bone health
[1] Dudley-Javoroski S, Saha PK, Liang G, Li C, Gao Z, and Shields RK. High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos Int 2012; 23:2335-2346.
[2] Goktepe A, Tugco I, Alaca, R, Gunduz S, Nikent M. Does standing protect bone density in patients with chronic spinal cord injury? JSCM 2008;31:197-201.
[3] Needham-Shropshire BM, Broton JG, Klose KJ, Lebwohl N, Guest RS, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 3. Lack of effect on bone mineral density. Arch Phys Med Rehabil 1997;78:799-803.
[4] Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993;74:73-78.
[5] Kaplan PE, Roden W, Gilbert E, Richards L, Goldschmidt JW. Reduction of hypercalciuria in tetraplegia after weight-bearing and strengthening exercises. Paraplegia 1981;19:289-293.
[6] Ben M, Harvey L, Denis S, et al. Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Aust J Physiother. 2005;51:251-256.
[7] de Bruin ED, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stussi E. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil 1999;80:214-220.
[8] Dudley-Javoroski S, and Shields RK. Active-resisted stance modulates regional bone mineral density in humans with spinal cord injury. Journal of Spinal Cord Medicine 2013; 36: 191-199.
Blood Pressure and Circulation
[1] Harkema SJ, Ferreira CK, van den Brand RJ, Krassioukov AV. Improvements in orthostatic instability with stand locomotor training in individuals with spinal cord injury. J Neurotrauma 2008;25:1467-1475.
Spasticity
[1] Odeen I, Knutsson E. Evaluation of the effects of muscle stretch and weight load in patients with spastic paraplegia. Scand J Rehabil Med 1981;13:117-21.
[2] Bohannon R. Tilt table standing for reducing spasticity after spinal cord injury. Arch Phys Med Rehabil 1993;74:1121-2.
[3] Kunkel C, Scremin A, Eisenberg B, Garcia J, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993;74:73-8.
[4] Dunn R, Walter J, Lucero Y, Weaver F, Langbein E, Fehr L, et al. Follow-up assessment of standing mobility device users. Assist Technol 1998;10:84-93.
[5] Eng JJ, Levins S, Townson A, Mah-Jones D, Bremner J, Huston G. Use of prolonged standing for individuals with spinal cord injuries. Phys Ther 2001;81:1392-9.
[6] Shields R & Dudley-Javoroski S. Monitoring standing wheelchair use after spinal cord injury: a case report. Disabil Rehabil 2005;27:142-6.
Bowel problems
[1] Hoenig H, Murphy T, Galbraith J, Zolkewitz M. Case study to evaluate a standing table for managing constipation. SCI Nursing 2001;18:74-7.
Other references
Dunn RB, Walter JS, Lucero Y, Weaver F, Langbein E, Fehr L, Johnson P, Riedy L. Follow-up assessment of standing mobility device users. Assist Technol. 1998;10(2):84-93.
Glickman LB, Geigle PR, Paleg GS. A systematic review of supported standing programs. J Pediatr Rehabil Med. 2010; 3(3),197-213.
Sadeghi M, McLvor J, Finlayson H, Sawatzky B. Static standing, dynamic standing and spasticity in individuals with spinal cord injury. Spinal Cord 2016;54:376-82.
Spinal Cord Injury Centre Physiotherapy Lead Clinicians. Clinical guideline for standing adults following spinal cord injury. 2019 Apr. Available from: https://scireproject.com/wp-content/uploads/2023/01/SCI-Standing-Clinical-Guidelines-Report-2019.pdf
Image credits
- 58/365 ©John Lustig, CC BY 2.0
- Image ©SCIRE
- Standing frame ©Memasa, CC BY-SA 3.0
- Functional Electrical Stimulation Therapy for walking ©MilosRPopovic, CC BY-SA 4.0
- Image ©SCIRE
- Modified from: oesteoporosis_eng ©go elsewhere…, CC BY-NC 2.0
- KRT LIFE HEALTH-BLOOD-PRESSURE PG ©Fort George G. Meade Public Affairs Office, CC BY 2.0
Disclaimer: This document does not provide medical advice. This information is provided for educational purposes only. Consult a qualified health professional for further information or specific medical advice. The SCIRE Project, its partners and collaborators disclaim any liability to any party for any loss or damage by errors or omissions in this publication.
Author: SCIRE Community Team | Reviewers: Tania Lam, Shannon Sproule | Published: 29 November 2017 | Updated: ~
Body weight supported treadmill training is a therapy that can be used to support walking training after spinal cord injury (SCI). This page outlines basic information about the use of body weight supported treadmill training after SCI.
Key Points
- Body weight supported treadmill training is a therapy modality in which part of a person’s body weight is supported while walking on a treadmill.
- It is usually used to work on walking ability, walking speed, and fitness in people with some control of movement in their legs after SCI (usually people with incomplete SCI).
- Research evidence supports that body weight supported treadmill training is effective to help improve walking in people with incomplete SCI. It may also have benefits for fitness, reducing spasticity, and overall wellness.
- The relationship between body weight supported treadmill training and stepping movements after complete SCI is not well understood. Further research is needed to understand whether body weight supported treadmill training has potential treatment benefits on walking (locomotor) function for people with complete injuries.
Body weight supported treadmill training is a therapy modality in which part of a person’s body weight is supported while walking on a treadmill. It is usually done using an overhead suspension system attached to a harness that supports part of a person’s body weight over a treadmill. While supported, the person walks with or without assistance from health providers on a treadmill.

Man engages in body weight supported treadmill training.
Body weight supported treadmill training is usually used to work on walking in people with some control of movement in their legs after SCI (usually people with incomplete SCI).
The goals of treatment with body weight supported treadmill training may include:
To practice walking and standing
Body weight supported treadmill training is usually used to work on walking and standing skills after incomplete SCI. Because the body weight is partially supported, walking can be practiced even when a person cannot stand or walk independently. This may also allow for walking training to begin earlier after injury.
To work on walking quality and speed
Body weight supported treadmill training may be used to practice better walking patterns and prevent unwanted movement compensations that can happen during unsupported walking. It may also allow a person to safely practice walking at faster speeds. This may provide important feedback to the nervous system to help with learning.
To train fitness and health
Standing upright and walking may have benefits for cardiovascular fitness and overall health. It may also have other benefits, such as improving spasticity and feelings of wellness.
Body weight supported treadmill training usually involves the use of an overhead harness and suspension system that supports the person in standing over a treadmill. There are other forms of body weight support training, such as underwater treadmills, anti-gravity treadmills and robotic assisted systems, although these are less common in standard clinical settings.
The amount of body weight that is supported will be different for each person depending on the characteristics of their SCI (such as the level of injury), the level of support provided by the health providers, and the person’s experience with the training.
Equipment

Equipment for a suspension type system includes a treadmill, overhead suspension system, and harness1
Body weight supported treadmill training may involve the use of several different pieces of equipment, depending on the type of support provided. The most common type of harness and suspension system may involve a variety of pieces of equipment such as:
- A harness
- Groin and abdominal straps and padding
- An overhead suspension system
- A treadmill with adjustable speeds
- A ramp up to the treadmill
- Additional tubing or strapping
- Parallel bars
- Braces and orthoses
Some body weight supported treadmill training systems may also involve the use of computer systems which control the training and/or robotic systems which guide movement of the legs.
Procedures
The exact procedures depend on the type of equipment used and the person’s physical abilities. General procedures for use of a standard harness and overhead suspension system may include the following steps:

Female clinician adjusting the harness, preparing a man for the treadmill.
- In order to ensure this treatment is safe for you, your health providers will measure your heart rate, blood pressure, and assess your risk of fractures before beginning this treatment.
- Your health providers with help you put the harness and groin straps on in a lying or standing position. The harness is then tightened so it does not slide up when weight is supported.
- The harness is then securely connected to the overhead suspension over the treadmill and you are lifted up using a mechanical lift to support some weight. There are usually bars on the side to hold on to for balance.
- Once you are standing upright, your health provider will then turn on the treadmill and gradually increase the speed of the treadmill. Depending on your needs and abilities, hands-on assistance or braces may be used to help move the legs or control the trunk and pelvis.
Training usually begins with maximum body weight support at a slow speed. The amount of support is usually between 35% and 50% of body weight, but depends on your ability to stand on one leg without it buckling. As you get used to the training, the amount of support provided is reduced and the speed or time spent on the treadmill can be increased. It is important to maintain a good quality walking pattern to practice normal movement patterns.
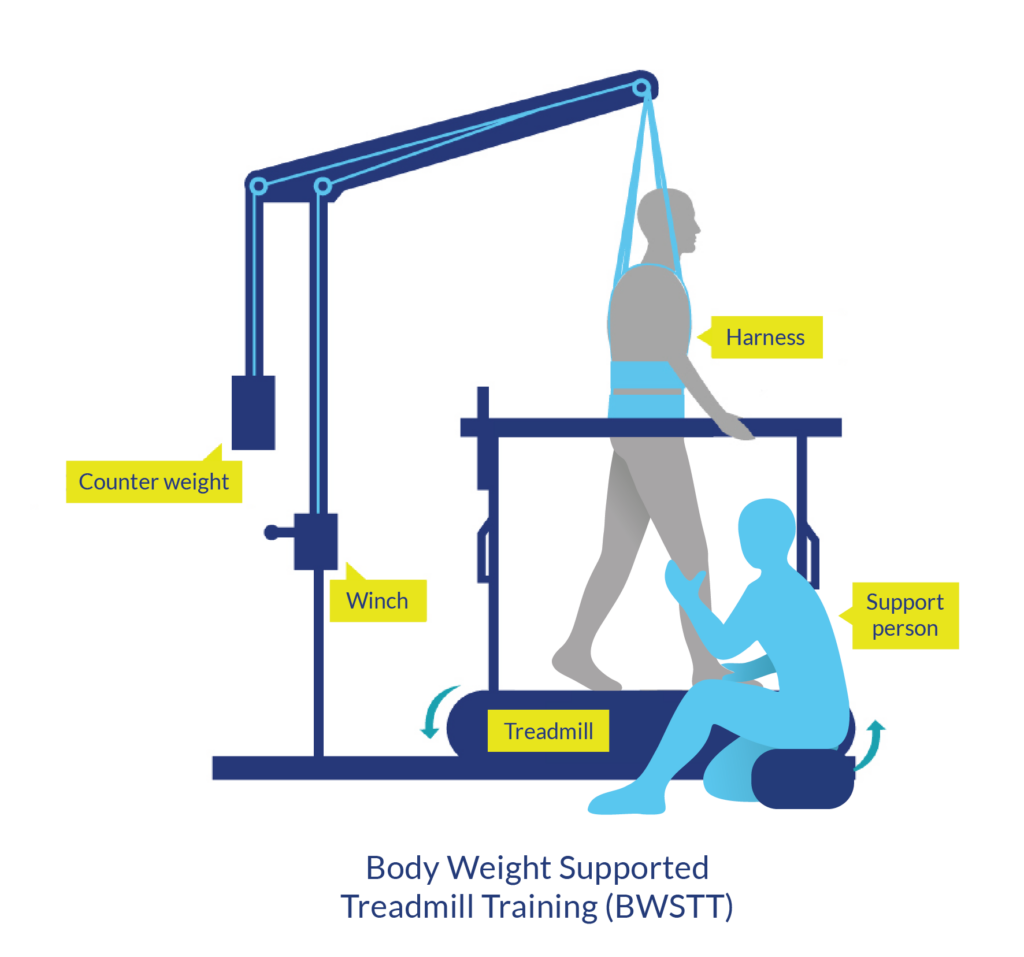
Body weight supported treadmill training is usually done using an overhead suspension system and harness that supports the body over a treadmill. Hands-on assistance or braces may be used to help move the legs or control the trunk and pelvis.
Amount of training
Your health provider will determine how long the training will last, depending on you and your training goals, as well as the availability of equipment and staff. Body weight supported treadmill training is often done for 15 to 30 minutes two to five times per week. However, we do not know what the optimal amount of training is.
Additional therapies
Body weight supported treadmill training is just one of many different walking therapies for people with SCI. It is often accompanied by other forms of walking training such as:
- Walking overground (off the treadmill) with or without an assistive device, such as a walker. This may be used to help reinforce walking after treadmill training in a form that is more realistic to everyday movement.

FES training can also strengthen muscles used for walking
- Functional electrical stimulation (FES) can be applied to the muscles of the legs and trunk during treadmill training to stimulate muscle activity. This may help to create stronger muscle contractions in weakened muscles when walking. Special FES systems (such as foot control systems that raise the toes up with each step) may be used to help with coordination when stepping.
It is important to speak with a health provider about body weight supported treadmill training to make sure it is safe and suitable for you and to learn how to use the equipment correctly.
Refer to our article on Functional Electrical Stimulation (FES) for more information!
There are some situations in which body weight supported treadmill training may be unsafe to use. This not a complete list, speak to a health provider about whether this treatment is safe and appropriate for you.
Body weight supported treadmill training should not be used in the following situations:
- By people with medical conditions where heart rate, blood pressure, or seizures are uncontrolled
- By individuals who are unable to stay upright for 5-10 minutes without a major drop in blood pressure
- By people at risk of broken bones (fractures), such as people with severe osteoporosis or recent fractures
- By people with joint limitations (such as contractures) which limit walking, weight-bearing, or standing
- In areas where the harness may put pressure on open wounds or areas at risk of pressure sores
- By people using mechanical ventilation
Body weight supported treadmill training should be used with caution in the following situations:
- When there are tubes or lines attached to the body, such as a feeding tube or indwelling catheter
- By people with severe and uncontrolled spasticity
- By people with blood clots or a history of blood clots
- By people with other major medical conditions or injuries
- By people prone to autonomic dysreflexia
There are some risks and side effects that should be discussed before participating in body weight supported treadmill training. This is not a complete list; ask your health providers for more detail.
Risks and side effects of body weight supported treadmill training may include:
- Groin discomfort or pain around the harness
- Skin irritation near where skin or clothes are shearing against the harness
- Abdominal discomfort or difficulty breathing if the harness is too tight
- Broken bones (fractures)
- Muscle strain, soreness, or injuries
- Worsening of muscle spasms
- Autonomic dysreflexia
- Changes in blood pressure that may cause light-headedness and dizziness
In addition to the risks and side effects of body weight supported treadmill training, there are also practical limitations its use, including:
- It is challenging to use and sometimes requires assistance from up to four people
- The equipment and staff time needed for body weight supported treadmill training can be very costly
- Many facilities do not have the staff or equipment to use body weight supported treadmill training in their day to day programs
Walking
Research studies have found that body weight supported treadmill training may help to:
- Improve walking ability in people with chronic incomplete SCI (weak evidence)
- Improve walking to a similar degree as walking off the treadmill at a similar intensity in people with recent incomplete SCI (moderate evidence)
- Improve functional walking in people with incomplete SCI when used together with functional electrical stimulation (FES) of the leg muscles (moderate evidence)

However, the benefits for walking do not appear to be unique to this type of training. Most walking strategies which involve weight-bearing (including walking overground, treadmill walking, and walking with FES) appear to be equally effective at improving walking after incomplete SCI.
Cardiovascular fitness
Several studies have looked at the effects of body weight supported treadmill training on different aspects of cardiovascular fitness after SCI. Taken altogether, these studies provide early evidence that body weight supported treadmill training helps to improve many aspects of cardiovascular fitness and health in people with complete and incomplete tetraplegia and paraplegia.
Other effects
In addition to benefits for walking and fitness, body weight supported treadmill training may also have other effects after SCI.
- Body weight supported treadmill training may help to improve spasticity (weak evidence)
- Body weight supported treadmill training may lead to greater life satisfaction and well-being (weak evidence)
- Body weight supported treadmill training has been thought to improve bone density after SCI, however, early research suggests that it may not help to prevent bone loss after SCI (weak evidence).
Although we tend to think about walking as being entirely voluntary, the ability to step and walk is actually related to both conscious and unconscious (automatic) processes. Some of the automatic walking processes are thought to be controlled within the spinal cord by networks of nerve cells known as central pattern generators or CPGs.
What are central pattern generators (CPGs)?
Central pattern generators (CPGs or spinal pattern generators) are networks of nerve cells in the spinal cord that generate rhythmic movement patterns. These networks do not require signals from the brain or sensation to keep going once they are activated.
CPGs were discovered when researchers found that animals with complete SCI demonstrated stepping movements when they were supported over a treadmill. These animals could not start the movement themselves, but once it was triggered (typically by electrical stimulation, application of certain drugs, or sensory stimulation to an area between the pubic bone and sacrum called the perineum), the stepping movements continued in a rhythmic pattern which resembled walking.
These networks of nerve connections are thought to be located within the spinal cord itself and exist to allow repetitive movements to continue without the need to think about each step.
Evidence for central pattern generators in humans with complete SCI
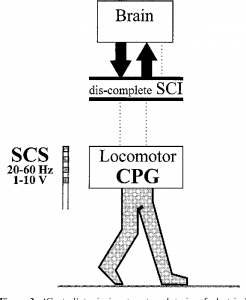
Generation of rhythmic movement through the CPG is debatable in people with SCI2
Researchers are still unsure about whether central pattern generators can be activated after complete SCI in humans. Researchers have suggested several observations that may show evidence of central pattern generators after complete SCI in humans, including:
- Spontaneous rhythmic movements below the level of injury;
- Stepping-like movements when electrical stimulation is applied through an electrode implanted over the spinal cord (epidural stimulation); and
- Rhythmic muscle contractions that can be induced through treatment with certain drugs.
However, there is debate among researchers about whether these findings really show evidence of central pattern generators or not. It is also not clear if central pattern generators are activated during body weight supported treadmill training after SCI.
Refer to our article on Epidural Stimulation for more information!
Automatic stepping is not walking
It is also important to consider that automatic stepping is not walking. Walking is much more complex, involving many other components, such as strength to support the body weight, balance to stay upright and shift weight, and sensation and voluntary control to adapt to the environment and situation. For these reasons, even if central pattern generators are activated after complete SCI, we do not know whether this will help a person regain walking ability or have any other benefits for functional walking.
Further research is needed to better understand central pattern generators after complete SCI. At this time, body weight supported treadmill training continues to be used clinically as a treatment for people with incomplete SCI who retain some movement in the legs.
Overall, the research evidence suggests that body weight supported treadmill training has positive effects on walking after incomplete SCI that are similar to other forms of walking training. It may also have benefits for fitness, spasticity, and wellness after SCI, although more high quality research is needed to confirm.
Body weight supported treadmill training appears to be relatively safe when used appropriately, however the equipment and support needed for this treatment may not be commonly available for regular use. If you are interested in this treatment, discuss your options with your health providers to find out if it is suitable to you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Professional “Lower Limb and Walking”, “Cardiovascular Health and Exercise”, “Bone Health”, “Mental Health” and “Spasticity” modules:
Lam T, Wolfe DL, Domingo A, Eng JJ, Sproule S (2014). Lower Limb Rehabilitation Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-74.
Available from: https://scireproject.com/evidence/lower-limb-and-walking/
Warburton DER, Krassioukov A, Sproule S, Eng JJ (2014). Cardiovascular Health and Exercise Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-48.
Available from: https://scireproject.com/evidence/cardiovascular-health-and-exercise/
Craven C, Lynch CL, Eng JJ (2014). Bone Health Following Spinal Cord Injury, In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-37.
Available from: https://scireproject.com/evidence/bone-health/
Orenczuk S, Mehta S, Slivinski J, Teasell RW (20140). Depression Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. Vancouver: p 1-35.
Available from: https://scireproject.com/evidence/mental-health/depression/
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Curt A, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0.
Available from: https://scireproject.com/evidence/spasticity/
Evidence for “Does body weight supported treadmill training improve walking after incomplete SCI?” is based on the following studies:
Walking
[1] Behrman AL, Ardolino E, VanHiel LR, Kern M, Atkinson D, Lorenz DJ, Harkema SJ. Assessment of functional improvement without compensation reduces variability of outcome measures after human spinal cord injury. Arch Phys Med Rehabil 2012;93:1518-29.
[2] Buehner JJ, Forrest GF, Schmidt-Read M, White S, Tansey K, Basso DM. Relationship between ASIA examination and functional outcomes in the NeuroRecovery Network Locomotor Training Program. Arch Phys Med Rehabil 2012;93:1530-40.
[3] Lorenz DJ, Datta S, and Harkema SJ. Longitudinal patterns of functional recovery in patients with incomplete spinal cord injury receiving activity-based rehabilitation. Arch Phys Med Rehabil 2012;93:1541-52.
[4] Winchester P, Smith P, Foreman N, Mosby J, Pacheco F, Querry R, and Tansey K. A prediction model for determining over ground walking speed after locomotor training in persons with motor incomplete spinal cord injury. J of Spinal Cord Med 2009;32:63-71.
[5] Hicks AL, Adams MM, Martin Ginis K, Giangregorio L, Latimer A, Phillips SM, and McCartney N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 2005;43:291-298.
[6] Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, and Hornby TG. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: A multicenter trial. Arch Phys Med Rehabil 2005;86:672-680.
[7] Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 2005;94:2844-2855.
[8] Protas EJ, Holmes SA, Qureshy H, Johnson A, Lee D, and Sherwood AM. Supported treadmill ambulation training after spinal cord injury: a pilot study. Arch Phys Med Rehabil 2001;82:825-831.
[9] Wernig A, Nanassy A, and Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord 1998;36:744-749.
[10] Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: A randomized clinical trial. Physical Therapy 2011;91:48-60.
[11] Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, and Scott M. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 2006;66:484-493.
[12] Hitzig SL, Craven BC, Panjwani A, Kapadia N, Giangregorio LM, Richards K, Masani K, and Popovic MR. Randomized trial of functional electrical stimulation therapy for walking in incomplete spinal cord injury: effects on quality of life and community participation. Top Spinal Cord Inj Rehabil 2013;19(4):245-58.
[13] Field-Fote EC, Lindley SD, and Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther 2005;29:127-137.
[14] Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther 2002;82:707-715.
[15] Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001;82:818-824.
[16] Hesse S, Werner C, and Bardeleben A. Electromechanical gait training with functional electrical stimulation: case studies in spinal cord injury. Spinal Cord 2004;42:346-352.
Cardiovascular fitness
[1] Miller PJ, Rakobowchuk M, Adams MM, Hicks AL, McCartney N, Macdonald MJ. Effects of short-term training on heart rate dynamics in individuals with spinal cord injury. Auton Neurosci 2009;150(1-2):116-21.
[2] Jack LP, Allan DB, Hunt KJ. Cardiopulmonary exercise testing during body weight supported treadmill exercise in incomplete spinal cord injury: a feasibility study. Technol Health Care 2009; 17(1):13-23.
[3] Soyupek F, Savas S, Ozturk O, Ilgun E, Bircan A, Akkaya A.E ffects of body weight supported treadmill training on cardiac and pulmonary functions in the patients with incomplete spinal cord injury. J Back Musculoskelet Rehabil 2009; 22(4):213-8.
[4] Ditor DS, Macdonald MJ, Kamath MV, Bugaresti J, Adams M, McCartney N, et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 2005b;43(11):664-73.
Other effects
[1] Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord 2005;43:649-657.
[2] Hicks AL, Adams MM, Martin GK, Giangregorio L, Latimer A, Phillips SM, McCartney N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: Effects on functional walking ability and measures of subjective well-being. Spinal Cord 2005;43:291-8.
[3] Adams M, Hicks A. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med 2011;34:488-94.
Evidence for “Can body weight supported treadmill training cause stepping after complete SCI?” is based on the following studies:
[1] Minassian K, Hofstoetter US, Dzeladini F, Guertin PA, Ijspeert A. The Human Central Pattern Generator for Locomotion. Neuroscientist. 2017 Mar 1:1073858417699790. doi: 10.1177/1073858417699790. [Epub ahead of print]
[2] Kern H, McKay WB, Dimitrijevic MM, Dimitrijevic MR. Motor control in the human spinal cord and the repair of cord function. Curr Pharm Des. 2005;11:1429-1439.
[3] Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626-2634.
[4] Guertin PA. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol. 2013 Feb 8;3:183.
[5] Yang JF, Gorassini M. Spinal and brain control of human walking: implications for retraining of walking. Neuroscientist. 2006 Oct;12(5):379-89.
[6] Illis LS. Is there a central pattern generator in man? Paraplegia. 1995 May;33(5):239-40.
Other references:
Body Weight Support Treadmill Training. Summary by: Lisa Taipalus BScPT, NEO Regional Stroke Best Practice Consultant for Physiotherapy, December 2009 (updated June 2011).
Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med 2011;364(21):2026-2036.
Senthilvelkumar T, Magimairaj H, Fletcher J, Tharion G, George J. Comparison of body weight-supported treadmill training versus body weight-supported overground training in people with incomplete tetraplegia: a pilot randomized trial. Clin Rehabil 2015; 29(1):42-49.
Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 2013;94(11):2297-2308.
Hicks AL, Ginis KA. Treadmill training after spinal cord injury: it’s not just about the walking. J Rehabil Res Dev 2008; 45(2):241-218.
Image credits:
- Image by SCIRE Community Team
- ‘Figure 1. Device for body weight support (LINAK, Silkeborg, Denmark) and treadmill (FITEX T-5050; Fitex, Gwangju, Korea) and treadmill’ from: Joon Lee B, Lee HJ, Lee, WH. The effects of intensive gait training with body weight support treadmill training on gait and balance in stroke disability patients: a randomized controlled trial. Phys Ther Rehabil Sci. 2013;2(2),104-110.
- Image by SCIRE Community Team
- Image by SCIRE Community Team
- Image by SCIRE Community Team
- Image by SCIRE Community Team
- ‘Figure 3 `Central’ tonic input, external train of electrical stimulation, delivered by SCS can induce stepping movements’ from: Pinter, M. M., & Dimitrijevic, M. R. (1999). Gait after spinal cord injury and the central pattern generator for locomotion. Spinal cord, 37(8), 531.
Disclaimer: This document does not provide medical advice. This information is provided for educational purposes only. Consult a qualified health professional for further information or specific medical advice. The SCIRE Project, its partners and collaborators disclaim any liability to any party for any loss or damage by errors or omissions in this publication.
Author: SCIRE Community Team | Reviewer: Holly Timms, Felicia Wong | Published: 21 November 2017 | Updated: ~
Spasticity is a common symptom of spinal cord injury (SCI) that causes movement problems and other symptoms. This page outlines basic information about spasticity and how it is treated after SCI.
Key Points
- Spasticity is a disorder of movement control that causes muscle spasms, increased muscle tone, and overactive reflexes. It can happen when the brain or spinal cord are damaged.
- Spasticity may cause problems with movement and posture, pain, fatigue, and many other symptoms. However, spasticity can also have benefits for movement and health.
- It is important to work together with your health team to decide whether your spasticity is problematic and worth treating.
- Treatment for spasticity usually begins with identifying specific triggers that make it worse. Management of these spasticity triggers along with other conservative treatments may alleviate these symptoms. Many of these treatments provide short-term relief of spasticity.
- Oral medications and botulinum toxin injections are also commonly used and effective for treating spasticity after SCI.

Spasticity is a movement control disorder that happens when the brain and spinal cord are damaged or do not develop properly. It is usually experienced as involuntary muscle spasms, increased muscle tone, and overactive reflexes.
Spasticity is a common symptom of SCI that can affect as many as three-quarters of people with SCI. It is more common among people with cervical and incomplete SCI. Spasticity can also be a symptom of other conditions like brain injury, stroke, and multiple sclerosis.
Spasticity can be experienced in many different ways depending on the person and the characteristics of their SCI.
Signs and symptoms of spasticity:
- Muscles that are constantly and involuntarily tensed (increased muscle tone)
- Stiff muscles that resist movement
- Muscle pain and fatigue
- Muscle spasms or jerky movements
- Uncontrolled movements or difficulty coordinating movements
- Exaggerated reflexes
- Altered posture or positioning
Spasticity is different from normal muscle tension because the amount of tension depends on the speed that the muscle is stretched. Faster movement speeds cause greater tension and resistance to movement.
Clonus
Clonus is often seen as rhythmic tapping or beating motion of the foot at the ankle.2
Clonus is a series of involuntary, rhythmic muscle contractions and relaxations, which often accompanies spasticity.
Clonus is most often seen in the ankle as a rhythmic tapping or beating motion of the foot that is triggered when there is stimulus to the ball of the foot. This can happen when putting weight onto the foot during transfers, standing, or walking. Clonus can also be experienced in other joints. Clonus can last for anywhere from a few seconds to several minutes.
Clonus is not the same as spasticity, but a related symptom that happens for similar reasons.
Spasticity may be constant or triggered by something
The symptoms of spasticity may be constant or come and go. They may also change over time. Some people will have muscle tension that is always present, while others will have spasticity that comes on or gets worse when it is triggered by something. Common spasticity triggers include:
- Movement of the arms or legs, especially quick movements
- Position changes, such as transfers, walking, or moving in bed
- Stretching
- Tight clothing or other discomfort below the level of injury
- Pressure sores, skin irritation, or wounds
- Bladder problems
- Bowel problems
- Cold temperatures
- Menstrual cycle or pregnancy
- Emotional or psychological stress
- Poor positioning in the wheelchair or bed
- Any other illnesses
A change in spasticity can be a sign of other health problems
Sudden or unexplained changes in spasticity can sometimes signal a health problem that needs attention – most commonly a bladder infection or skin breakdown. If you are not sure why your spasticity has changed, speak to your health providers for further testing.
Spasticity is related to several changes to the body that happen after SCI. The main reason for spasticity after SCI is a reduced ability of the brain to ‘calm down’ overactive reflexes. Over time, the muscles and tendons may also change, becoming more tense and stiff, which also contributes to the symptoms of spasticity.
The stretch reflex
The stretch reflex is an automatic movement response that happens when a muscle is stretched quickly, causing the muscle to tense. It is commonly tested as the ‘tendon tap’ below the kneecap.
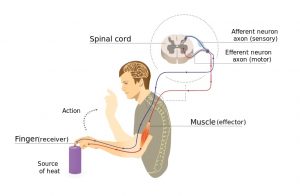
Pain signals from touching something hot travels to the spinal cord and back to the muscles without going to the brain first.4
When a muscle is quickly stretched, it activates special stretch sensors called muscle spindles. They send a signal through sensory neurons to the spinal cord. In the spinal cord, the message is passed along to motor neurons, which send a movement command back to the muscle, causing it to contract. This reflex happens in the spinal cord without travelling to the brain first.
Like muscle stretch, pain can also trigger spinal cord reflexes that use the same nerve pathway as the stretch reflex. For example, stepping on something sharp or touching a hot burner activates spinal cord reflexes.
The brain normally dampens spinal cord reflexes
Although the stretch reflex happens in the spinal cord, the brain influences how sensitive the reflex is. The brain normally sends signals down the spinal cord, which dampens the sensitivity of reflexes.
This is called descending inhibition. ‘Descending’ means ‘coming down from the brain’, and ‘inhibition’ means ‘reducing the activity of’ the stretch reflex. Descending inhibition is important because it tells the stretch reflex to ‘calm down’ so it doesn’t get in the way of normal movements.
Spinal cord injury prevents the brain from dampening spinal cord reflexes
When the spinal cord is injured, descending inhibition from the brain is cut off. Without its calming effects,
the stretch reflex becomes overactive. This can lead to a constant level of muscle tension (called muscle tone) and excessive reflexes which cause the muscles to tighten uncontrollably or unexpectedly and the other symptoms of spasticity.
Watch SCIRE’s YouTube video explaining why spasticity happens after SCI.5
The main way that spasticity is diagnosed and monitored is through a physical examination. Your health providers will talk to you about your symptoms, functional abilities, and current treatment plan, look at your muscles and posture, and test the muscles in various ways. This may include:
- Hands-on tests where the joints are moved slowly and quickly
- Active tests of strength and movement
- Testing your reflexes
Your health providers may also observe tasks like walking, transferring, and eating – this can help them understand how spasticity affects you in your everyday life.
Health providers often use special collections of questions and tests called outcome measures, which help them accurately keep track of changes in spasticity. Spasticity may change over time so regular check-ins with your health team, especially while figuring out what works best for you, are often an important part of managing your spasticity.
Spasticity can negatively affect the health and wellness of some people, but it can sometimes have benefits as well. It is important to determine whether your spasticity is a problem for you. Treating spasticity unnecessarily can have drawbacks, such as unwanted side effects, costs, and time. It is important that you discuss your treatment options and weigh the pros and cons of treating spasticity together with your health team to determine the best course of action for you.
Problems with spasticity
 Muscle spasms and reflexes can contribute to a number of potential problems, such as:
Muscle spasms and reflexes can contribute to a number of potential problems, such as:
- Pain
- Sleep problems
- Reduced mobility and function
- Difficulties maintaining posture and positioning
- Skin breakdown and hygiene concerns
- Bladder and bowel accidents
- Joint contractures
- Sexual and reproductive health issues
- Difficulties with care
Benefits of spasticity
Spasticity can also have some benefits for people with SCI, which may include:
- Better mobility, standing, and walking
- Assistance with transfers (such as supporting the body weight while transferring from a wheelchair to a bed or chair)
- Preventing muscle wasting or weakening due to inactivity
- Improved circulation
- Intentionally triggered spasms can help to empty the bowel and bladder in people with certain types of bowel or bladder problems
- Reflex erections during sexual activity
- It may serve as a warning sign of infections or other health issues
YouTube video about the downfalls of treating non-problematic spasticity.8
There are many different treatments for spasticity. Every person’s spasticity is different, so finding the best treatment or combination of treatments often involves trial and error.
Spasticity treatment usually starts with conservative treatments such as positioning and maintaining good muscle length. If these do not provide enough relief, spasticity medications and injections may be recommended. Surgical treatments are considered as a last option for severe spasticity.
Avoiding spasticity triggers
An important part of managing spasticity is learning how to manage your spasticity triggers. Spasticity is often triggered by bladder, bowel, skin, or other health issues, so maintaining good overall health and taking care of these issues is an important part of managing spasticity. Speak to your health providers about optimizing your self-care routines to prevent spasticity.
Movement and therapeutic treatment options
There are a number of different movement, hands-on, and electrical treatments that may be done on your own, with a caregiver, or in conjunction with a therapist. These treatments generally produce fewer side effects than medications or surgeries; however, they also tend to have short-term effects.
Posture and positioning
Good posture and positioning may help to keep the muscles at an appropriate length and help prevent contractures. You may need to work with your health providers to determine the best positions and equipment to manage your spasticity.
Stretching and range of motion
Stretching and range of motion exercises are commonly used treatments to reduce spasticity and minimize complications like contractures after SCI. Stretching is often achieved through prolonged positioning, such as placing a wedge between the knees to stretch the hips.
Bracing and casting
 Various braces, orthoses, and casts may be used to maintain proper positioning of the arms and legs to help reduce spasticity, improve function, and prevent complications.
Various braces, orthoses, and casts may be used to maintain proper positioning of the arms and legs to help reduce spasticity, improve function, and prevent complications.
Standing
Standing can provide a prolonged stretch to certain muscles, such as the calf and hamstring muscles, which may help with spasticity. For some people, standing may be done using specialized equipment such as tilt tables, standing frames, and standing wheelchairs.
Neurodevelopmental therapy (NDT)
Neurodevelopmental therapy (NDT, sometimes called Bobath therapy) is a type of physiotherapy and occupational therapy treatment where a therapist uses hands-on techniques to guide a person through movements. It is used to help practice quality functional movements.
Walking
 Walking may be done by some people (typically with incomplete SCI) with or without gait aids or assistance from health providers or with the use of specialized equipment such as body weight supported treadmill training or robotic exoskeletons.
Walking may be done by some people (typically with incomplete SCI) with or without gait aids or assistance from health providers or with the use of specialized equipment such as body weight supported treadmill training or robotic exoskeletons.
Functional electrical stimulation (FES) exercise
Functional electrical stimulation (FES) involves the use of electrical stimulation to activate specific muscles of the arms or legs during an activity such as stationary cycling, arm exercises or walking.
See our article on FES for more information!
Massage
Massaging muscles may help to stimulate the sensory nerves, which are part of the reflex spasticity response.
Transcutaneous electrical nerve stimulation (TENS)
Transcutaneous electrical nerve stimulation (TENS) involves the use of electrodes placed on the skin to stimulate the sensory nerves without producing muscle tension.
See our article on TENS for more information!
Do movement and therapeutic treatment options work?
Although many of these treatments are commonly used in the treatment of spasticity, the research is unclear about whether a number of these therapies, including stretching and range of motion, standing, neurodevelopmental therapy, and massage; are actually effective for reducing spasticity after SCI. However, many of these treatments often have several therapeutic purposes after SCI (such as reducing pain or preventing contractures), which may explain their widespread use. Further research is needed to better understand the effects of these treatments on spasticity.
However, there is evidence that body weight supported treadmill training, robotic exoskeleton walking, functional electrical stimulation (FES) exercise, and TENS are effective treatments for reducing spasticity after SCI.
Medications
Oral medications are typically prescribed for the treatment of widespread spasticity. Finding the right medication may involve trial and error and involves working closely with your doctor to find the best fit for you.
Baclofen (tablets and baclofen pumps)
Baclofen (Lioresal) is a muscle relaxant that is commonly used to treat spasticity. It can be taken as tablets by mouth or administered into the sac surrounding the spinal cord (called intrathecal baclofen) through a surgically implanted baclofen pump. Baclofen is effective for treating spasticity after SCI. However, it can have several side effects such as dizziness, drowsiness, anxiety, confusion, and weakness. Extra care is also needed when discontinuing therapy to avoid withdrawal symptoms. Baclofen is the most common medication prescribed for spasticity after SCI.
Baclofen is commonly used for controlling spasticity after a spinal cord injury. This can be achieved by surgically implementing a pump that connects directly to the spinal cord.11
Watch “Intrathecal Baclofen for Reducing Spasticity After Spinal Cord Injury” from our SCIRE video series on Neuromodulation!
Other spasticity medications
A number of other medications are used clinically or have been studied for their effects on spasticity after SCI. Speak to your doctor or pharmacist for more information about these medications.
Medications that are effective for spasticity after SCI:
Medications that may be effective for spasticity after SCI:
- Cannabinoid medications (Dronabinol and Nabilone)
- Gabapentin
- Orphenadrine Citrate
- Diazepam
- Dantrolene
Medications that are not supported for treating spasticity after SCI:
- Fampridine (4-Aminopyridine)
- Levetiracetam
Injections
Injections into the nerves and muscles may be used to help manage localized areas of spasticity.
Botulinum toxin (Botox) injections
 Botulinum toxin is a toxin that can cause muscle paralysis. Very small doses of certain strains of botulinum toxin can be injected into muscles to treat spasticity. It is commonly known for its cosmetic use by its trade names Botox, Dysport, and Xeomin. Botulinum toxin injections are temporary, with effects that wear off over time (usually around 3 to 6 months). Research evidence supports that botulinum toxin is effective in reducing focal spasticity after SCI.
Botulinum toxin is a toxin that can cause muscle paralysis. Very small doses of certain strains of botulinum toxin can be injected into muscles to treat spasticity. It is commonly known for its cosmetic use by its trade names Botox, Dysport, and Xeomin. Botulinum toxin injections are temporary, with effects that wear off over time (usually around 3 to 6 months). Research evidence supports that botulinum toxin is effective in reducing focal spasticity after SCI.
See our article on Botulinum Toxin for more information
Phenol injections
Phenol injections involve injecting a type of alcohol into nerves which supply the spastic muscle. Phenol damages the nerve axons, so the nerves cannot send signals to the muscles that cause spasticity. This procedure is also sometimes used with another alcohol, ethanol. Phenol injections may be effective for reducing spasticity after SCI.
Surgical treatments
 Surgery is typically reserved for and joint contractures are impacting care, function and quality of life
Surgery is typically reserved for and joint contractures are impacting care, function and quality of life
Tendon releases or transfers
Tendon releases are surgeries that lengthen shortened tendons (the part of the muscle that attaches to a bone) affected by spasticity. Tendon transfers involve surgically moving tendons that attach to muscles. These techniques can assist with better positioning of the feet or arms when excessive spasticity interferes with safe or appropriate positioning. However, there is limited research investigating the specific effects with SCI.
Myelotomy and Rhizotomy
Myelotomy and rhizotomy are surgical procedures that involve intentionally damaging part of the spinal cord (myelotomy) or nerve (rhizotomy) to reduce spasticity. Damaging the nerve fibers related to spasticity can prevent them from communicating and causing unwanted muscle spasms. These techniques are not common because they are permanent and invasive. They are only used for severe and intolerable spasticity that does not respond to other treatments. Myelotomy is effective for reducing spasticity after SCI.
Other treatments
A number of other medical, alternative, and self-management treatments may be used to manage spasticity. There is some limited evidence that other treatments, such as transcranial magnetic stimulation (TMS), hippotherapy (therapeutic horseback riding), and others may help to treat spasticity after SCI. However, these treatments are not typically used or available in standard practice at this time. Speak with your health providers about any treatments you are considering trying as a treatment for your spasticity.
The research evidence suggests that conservative treatments that involve active movement and electrical stimulation help to reduce spasticity short-term after SCI. It is not clear whether passive movement therapies like stretching help treat spasticity.
Medications and injections may include baclofen and botulinum toxin injections, which are effective for treating spasticity but may have additional side effects. There are many other drugs and treatments that may require further research. Surgery may be considered as a last resort if other treatments fail.
It is important to discuss any questions of concerns that you have about your treatment options in detail with your health providers to find the best management options for you.
For a review of how we assess evidence at SCIRE Community and advice on making decisions, please see SCIRE Community Evidence.
Parts of this page have been adapted from the SCIRE Professional “Spasticity” Module:
Hsieh JTC, Connolly SJ, McIntyre A, Townson AF, Short C, Mills P, Vu V, Benton B, Wolfe DL (2016). Spasticity Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Curt A, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Version 6.0.
Available from: https://scireproject.com/evidence/spasticity/
Evidence for “Movement and physical therapies” is based on the following studies:
Stretching and range of motion
[1] Skold C. Spasticity in spinal cord injury: self-and clinically rated intrinsic fluctuations and intervention-induced changes. Arch Phys Med Rehabil 2000;81:144-9.
[2] Kakebeeke T, Lechner H, Knapp P. The effect of passive cycling movements on spasticity after spinal cord injury: preliminary results. Spinal Cord 2005;43:483-8.
Standing
[1] Odeen I & Knutsson E. Evaluation of the effects of muscle stretch and weight load in patients with spastic paraplegia. Scand J Rehabil Med 1981;13:117-21.
[2] Short-term effects of surface electrical stimulation. Arch Phys Med Rehabil 1988b;69:598-604.
[3] Adams M, Hicks A. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med 2011;34:488-94.
Neurodevelopmental Therapy
[1] Li S, Xue S, Li Z, Liu X. Effect of baclofen combined with neural facilitation technique on the reduction of muscular spasm in patients with spinal cord injury. Neur Regen Res 2007;2:510-2.
Walking
[1] Fang C, Hsu M, Chen C, Cheng H, Chou C, Chang Y. Robot-assisted passive exercise for ankle hypertonia in individuals with chronic spinal cord injury. J Med Biol Eng 2015;35:464-72.
[2] Mirbagheri M, Kindig M, Niu X. Effects of robotic-locomotor training on stretch reflex function and muscular properties in individuals with spinal cord injury. Clinical Neurophysiol 2015;126:997-1006.
[3] Manella K & Field-Fote E. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci 2013;31:633-46.
Functional Electrical Stimulation
[1] Kapadia N, Masani K, Craven B, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med 2014;37:511-24.
[2] Manella K & Field-Fote E. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci 2013;31:633-46.
[3] Ralston K, Harvey L, Batty J, et al. Functional electrical stimulation cycling has no clear effect on urine output, lower limb swelling, and spasticity in people with spinal cord injury: A randomised cross-over trial. J Physiother 2013;59:237-43.
[4] Kuhn D, Leichtfried V, Schobersberger W. Four weeks of functional electrical stimulated cycling after spinal cord injury: a clinical cohort study. Inter J Rehabil Res 2014;37:243-50.
[5] Sadowsky C, Hammond E, Strohl A, et al. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med 2013;36:623-31.
[6] Reichenfelser W, Hackl H, Hufgard J, Kastner J, Gstaltner K, Gföhler M. Monitoring of spasticity and functional ability in individuals with incomplete spinal cord injury with a functional electrical stimulation cycling system. J Rehabil Med 2012;44:444-9.
[7] Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil 2008;22:627-34
[8] Mirbagheri M, Ladouceur M, Barbeau H, Kearney R. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. Trans Neural Syst Rehabil Eng 2002;10:280-9.
[9] Granat M, Ferguson A, Andrews B, Delargy M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury–observed benefits during gait studies. Paraplegia 1993;31:207-15.
[10] Thoumie P, Le C, Beillot J, Dassonville J, Chevalier T, Perrouin-Verbe B et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia 1995;33:654-9.
Transcutaneous Electrical Nerve Stimulation
[1] Oo W. Efficacy of addition of transcutaneous electrical nerve stimulation to standardized physical therapy in subacute spinal spasticity: a randomized controlled trial. Arch Phys Med Rehabil 2014;95:2013-20.
[2] Chung B & Cheng, B. Immediate effect of transcutaneous electrical nerve stimulation on spasticity in patients with spinal cord injury. Clinical Rehabilitation 2010;24:202-210.
[3] Aydin G, Tomruk S, Keleş I, Demir SO, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil. 2005 Aug;84(8):584-92.
Massage
[1] Goldberg J, Seaborne D, Sullivan S, Leduc B. The effect of therapeutic massage on H-reflex amplitude in persons with a spinal cord injury. Phys Ther 1994;74:728-37.
Evidence for “Medications” is based on the following studies:
Baclofen
[1] Chu V, Hornby T, Schmit B. Effect of antispastic drugs on motor reflexes and voluntary muscle contraction in incomplete spinal cord injury. Arch Phys Med Rehabil 2014;95:622-32.
[2] Nance P, Huff F, Martinez-Arizala A, Ayyoub Z, Chen D, Bian A, Stamler D. Efficacy and safety study of arbaclofen placarbil in patients with spasticity due to spinal cord injury. Spinal Cord 2011;49:974-80.
[3] Aydin G, Tomruk S, Keles I, Demir S, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil 2005;84:584-92.
[4] Duncan G, Shahani B, Young R. An evaluation of baclofen treatment for certain symptoms in patients with spinal cord lesions. A double-blind, cross-over study. Neurology 1976;26:441-6.
[5] Burke D, Gillies J, Lance J. An objective assessment of a gamma aminobutyric acid derivative in the control of spasticity. Proc Aust Assoc Neurol 1971;8:131-4.
[6] Dicpinigaitis P, Allusson V, Baldanti A, and Nalamati J. Ethnic and gender differences in cough reflex sensitivity. Respiration 2001;68:480-2.
[7] Veerakumar A, Cheng J, Sunshine A, Ye X, Zorowitz R, Anderson W. Baclofen dosage after traumatic spinal cord injury: a multi-decade retrospective analysis. Clin Neurol Neurosurg 2015;129:50-6.
[8] Nance P. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc 1994;17:150-6.
Intrathecal Baclofen
[1] Ordia J, Fischer E, Adamski E, Spatz E. Chronic intrathecal delivery of baclofen by a programmable pump for the treatment of severe spasticity. J Neurosurg 1996;85:452-7.
[2] Nance P, Schryvers O, Schmidt B, Dubo H, Loveridge B, Fewer D. Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci 1995;22:22-9.
[3] Coffey J, Cahill D, Steers W, Park T, Ordia J, Meythaler J, et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg 1993;78:226-32.
[4] Hugenholtz H, Nelson R, Dehoux E, Bickerton R. Intrathecal baclofen for intractable spinal spasticity-a double-blind cross-over comparison with placebo in 6 patients. Can J Neurol Sci 1992;19:188-95.
[5] Loubser P, Narayan R, Sandin K, Donovan W, Russell K. Continuous infusion of intrathecal baclofen: long-term effects on spasticity in spinal cord injury. Paraplegia 1991;29:48-64.
[6] Penn R, Savoy S, Corcos D, Latash M, Gottlieb G, Parke B et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989;320:1517-21.
[7] Boviatsis E, Kouyialis A, Korfias S, Sakas D. Functional outcome of intrathecal baclofen administration for severe spasticity. Clin Neurol Neurosurg 2005;107:289-95.
[8] Azouvi P, Mane M, Thiebaut J, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil 1996;77:35-9.
[9] Plassat R, Perrouin Verbe B, Menei P, Menegalli D, Mathe J, Richard I. Treatment of spasticity with intrathecal baclofen administration: Long-term follow-up review of 40 patients. Spinal Cord 2004;42:686-93.
[10] Zahavi A, Geertzen J, Middel B, Staal M, Rietman J. Long term effect (more than five years) of intrathecal baclofen on impairment, disability, and quality of life in patients with severe spasticity of spinal origin. J Neurol Neurosurg Psychi 2004;75:1553-7.
[11] Korenkov A, Niendorf W, Darwish N, Glaeser E, Gaab M. Continuous intrathecal infusion of baclofen in patients with spasticity caused by spinal cord injuries. Neurosurg Rev 2002;25:228-30.
[12] Broseta J, Garcia-March G, Sanchez-Ledesma M, Anaya J, Silva I. Chronic intrathecal baclofen administration in severe spasticity. Stereotact Funct Neurosurg 1990;54-55:147-53.
[13] Parke B, Penn R, Savoy S, Corcos D. Functional outcome after delivery of intrathecal baclofen. Arch Phys Med Rehabil 1989;70:30-2.
Tizanidine
[1] Chu et al. 2014.
[2] Nance P, Bugaresti J, Shellenberger K, Sheremata W, Martinez-Arizala A. Efficacy and safety of tizanidine in the treatment of spasticity in patients with spinal cord injury. Neurol 1994;44:S44-51.
[3] Mirbagheri M, Kindig M, Niu X, Varoqui D. Therapeutic effects of anti-spastic medication on neuromuscular abnormalities in SCI: A system identification approach. IEEE EMBS 2013;6203-6.
Clonidine
[1] Stewart J, Barbeau H, Gauthier S. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Can J Neurol Sci 1991;18:321-32.
[2] Malinovsky J, Malinge M, Lepage J, Pinaud M. Sedation caused by clonidine in patients with spinal cord injury. Bri J Anaesthesia 2003;90:742-5.
[3] Remy-Neris O, Barbeau H, Daniel O, Boiteau F, Bussel B. Effects of intrathecal clonidine injection on spinal reflexes and human locomotion in incomplete paraplegic subjects. Exp Brain Res 1999;129:433-40.
4-Aminopyridine
[1] Cardenas D, Ditunno J, Graziani V, Jackson A, Lammertse D, Potter P, et al. Phase 2 trial of sustained-release fampridine in chronic spinal cord injury. Spinal Cord 2007;45:158-68.
[2] Cardenas D, Ditunno JF, Graziani V, et al. Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal Cord 2014;52:70-76.
[3] Potter P, Hayes K, Segal J, Hsieh J, Brunnemann S, Delaney G, et al. Randomized double-blind crossover trial of fampridine-SR (sustained release 4-aminopyridine) in patients with incomplete spinal cord injury. J Neurotrauma 1998a;15:837-49.
[4] Potter P, Hayes K, Hsieh J, Delaney G, Segal J. Sustained improvements in neurological function in spinal cord injured patients treated with oral 4-aminopyridine: Three cases. Spinal Cord 1998b;36:147-55.
[5] Donovan W, Halter J, Graves D, Blight A, Calvillo O, McCann M, et al. Intravenous infusion of 4-AP in chronic spinal cord injured subjects. Spinal Cord 2000;38:7-15.
[6] Hayes K, Potter P, Wolfe D, Hsieh J, Delaney G, Blight AR. 4-Aminopyridine-sensitive neurologic deficits in patients with spinal cord injury. J Neurotrauma 1994;11:433-46.
Cyproheptadine
[1] Thompson C, Hornby T. Divergent modulation of clinical measures of volitional and reflexive motor behaviors following serotonergic medications in human incomplete spinal cord injury. J Neurotrauma. 2013;30:498-502.
[2] Nance et al. 1994.
[3] Meythaler J, Roper J, Brunner R. Cyproheptadine for intrathecal baclofen withdrawal. Arch Phys Med Rehabil 2003;84:638-42.
Gabapentin
[1] Gruenthal M, Mueller M, Olson W, Priebe M, Sherwood A, Olson W. Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord 1997;35:686-9.
Orphenadrine Citrate
[1] Casale R, Glynn C, Buonocore M. Reduction of spastic hypertonia in patients with spinal cord injury: a double-blind comparison of intravenous orphenadrine citrate and placebo. Arch Phys Med Rehabil 1995;76:660-5.
Cannabinoids
[1] Pooyania S, Ethans K, Szturm T, Casey A, Perry D. A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury. Arch Phys Med Rehabil 2010;91:703-7.
[2] Hagenbach U, Luz S, Ghafoor N, Berger J, Grotenhermen F, Brenneisen R, et al. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord 2007;45:551-62.
[3] Kogel R, Johnson P, Chintam R, Robinson C, Nemchausky B. Treatment of spasticity in spinal cord injury with dronabinol, a tetrahydrocannabinol derivative. Am J Ther 1995;2:799-805.
Evidence for “Injections” is based on the following studies:
[1] Richardson D, Sheean G, Werring D, Desai M, Edwards S, Greenwood R et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychi 2000;69:499-506.
[2] Spiegl U, Maier D, Gonschorek O, Heyde C, Buhren V. Antispastic therapy with botulinum toxin type A in patients with traumatic spinal cord lesion. GMS Interdiscip Plast Reconstr Surg 2014;3:1-5.
[3] Bernuz B, Genet F, Terrat P, et al. Botulinum toxin effect on voluntary and stretch reflex-related torque produced by the quadriceps: An isokinetic pilot study. Neurorehabil Neural Repair 2012;26:542-7.
[4] Hecht M, Stolze H, uf dem B, Giess R, Treig T, Winterholler M et al. Botulinum neurotoxin type A injections reduce spasticity in mild to moderate hereditary spastic paraplegia–report of 19 cases. Mov Disord 2008;23:228-33.
[5] Uchikawa K, Toikawa H, Liu M. Subscapularis motor point block for spastic shoulders in patients with cervical cord injury. Spinal Cord 2009;47:249-51.
[6] Ghai A, Sangwan S, Hooda S, Garg N, Kundu Z, Gupta T. Evaluation of interadductor approach in neurolytic blockade of obturator nerve in spastic patients. Saudi J Anaesth 2013;7:420-6.
[7] Ghai A, Sangwan S, Hooda S, Kiran S, Garg N. Obturator neurolysis using 65% alcohol for adductor muscle spasticity. Saudi J Anaesth 2012;6:282-4.
[8] Yasar E, Tok F, Taskaynatan M, Yilmaz B, Balaban B, Alaca R. The effects of phenol neurolysis of the obturator nerve on the distribution of buttock-seat interface pressure in spinal cord injury patients with hip adductor spasticity. Spinal Cord 2010;48:828-31.
Evidence for “Surgeries” is based on the following studies:
[1] Livshits A, Rappaport Z, Livshits V, Gepstein R. Surgical treatment of painful spasticity after spinal cord injury. Spinal Cord 2002;40:161-6.
[2] Putty T & Shapiro S. Efficacy of dorsal longitudinal myelotomy in treating spinal spasticity: a review of 20 cases. J Neurosurg 1991;75:397-401.
Other references:
Abel NA, Smith RA. Intrathecal baclofen for treatment of intractable spinal spasticity. Arch Phys Med Rehabil 1994; 75:54-58.
Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord 2005;43(10):577-586.
Al-Khodairy AT, Gobelet C, Rossier AB. Has botulinum toxin type A a place in the treatment of spasticity in spinal cord injury patients? Spinal Cord 1998; 36(12):854-858.
Avellino AM, Loeser JD. Intrathecal baclofen for the treatment of intractable spasticity of spine or brain etiology. Neuromodulation 2000; 3(2):75-81.
Bajd T, Gregoric M, Vodovnik L, Benko H. Electrical stimulation in treating spasticity resulting from spinal cord injury. Arch Phys Med Rehabil 1985; 66(8):515-517.
Bakheit AM, Pittock S, Moore AP, Wurker M, Otto S, Erbguth F et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. Eur J Neurol 2001; 8(6):559-565.
Bohannon RW. Tilt table standing for reducing spasticity after spinal cord injury. Arch Phys Med Rehabil 1993; 74(10):1121-1122.
Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine 2001; 26(24 Suppl):S146-S160.
Canadian Paraplegic Association. Workplace participation national survey of Canadians with SCI. Canadian Paraplegic Association http://www.canparaplegic.org/en/Employment_and_Education_24/EMPLOYMENT_6/14.html. 1996; Last accessed: 9-15-2008.
Corry IS, Cosgrove AP, Walsh EG, McClean D, Graham HK. Botulinum toxin A in the hemiplegic upper limb: a double-blind trial. Dev Med Child Neurol 1997; 39(3):185-193.
Elbasiouny, S. M., Moroz, D., Bakr, M. M., & Mushahwar, V. K. (2010). Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabilitation and neural repair, 24(1), 23-33.
Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord 2008; 46(2):96-102.
Goulet C, Arsenault AB, Bourbonnais D, Laramee MT, Lepage Y. Effects of transcutaneous electrical nerve stimulation on H-reflex and spinal spasticity. Scand J Rehabil Med 1996; 28(3):169-176.
Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl 1997; 6:S92-120.
Granat MH, Ferguson AC, Andrews BJ, Delargy M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury–observed benefits during gait studies. Paraplegia 1993; 31(4):207-215.
Gruenthal M, Mueller M, Olson WL, Priebe MM, Sherwood AM, Olson WH. Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord 1997; 35(10):686-689.
Hayes KC. Fampridine-SR for multiple sclerosis and spinal cord injury. Expert Rev Neurother 2007; 7(5):453-461.
Heetla HW, Staal MJ, Kliphuis C, van Laar T. The incidence and management of tolerance in intrathecal baclofen therapy. Spinal Cord, 2009; 47:751-756.
Heetla HW, Staal MJ, Kliphuis C, van Laar T. Tolerance to continuous intrathecal baclofen infusion can be reversed by pulsatile bolus infusion. Spinal Cord, 2010; 48: 483-486.
Hidler, J. M., & Rymer, W. Z. (1999). A simulation study of reflex instability in spasticity: origins of clonus. IEEE Transactions on Rehabilitation Engineering, 7(3), 327-340.
Hinderer SR, Lehmann JF, Price R, White O, deLateur BJ, Deitz J. Spasticity in spinal cord injured persons: quantitative effects of baclofen and placebo treatments. Am J Phys Med Rehabil 1990; 69(6):311-317.
Hinderer SR. The supraspinal anxiolytic effect of baclofen for spasticity reduction. Am J Phys Med Rehabil 1990; 69(5):254-258.
Hyman N, Barnes M, Bhakta B, Cozens A, Bakheit M, Kreczy-Kleedorfer B et al. Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry 2000; 68(6):707-712.
Kesiktas N, Paker N, Erdogan N, Gulsen G, Bicki D, Yilmaz H. The use of hydrotherapy for the management of spasticity. Neurorehabil Neural Repair 2004; 18(4):268-273.
Kirshblum S. Treatment alternatives for spinal cord injury related spasticity. J Spinal Cord Med 1999; 22(3):199-217.
Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD et al. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med 2005; 28(3):241-245.
Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clinical Rehabilitation 2008; 22(7):627-634.
Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993; 74:73-78.
Lechner HE, Feldhaus S, Gudmundsen L, Hegemann D, Michel D, Zach GA et al. The short-term effect of hippotherapy on spasticity in patients with spinal cord injury. Spinal Cord 2003; 41(9):502-505.
Levi R, Hultling C, Nash MS, Seiger A. The Stockholm spinal cord injury study: 1. medical problems in a regional SCI population. Paraplegia 1995; 33:308-315.
Maurer M, Henn V, Dittrich A, Hofmann A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. Eur Arch Psychiatry Clin Neurosci 1990; 240(1):1-4.
Maynard FM, Karunas RS, Waring WP, III. Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 1990; 71(8):566-569.
Midha M, Schmitt JK. Epidural spinal cord stimulation for the control of spasticity in spinal cord injury patients lacks long-term efficacy and is not cost-effective. Spinal Cord 1998; 36(3):190-192.
Mirbagheri MM, Ladouceur M, Barbeau H, Kearney RE. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. IEEE Trans Neural Syst Rehabil Eng 2002; 10(4):280-289.
Nance PW, Shears AH, Nance DM. Reflex changes induced by clonidine in spinal cord injured patients. Paraplegia 1989; 27(4):296-301.
Ochs G, Struppler A, Meyerson BA, Linderoth B, Gybels J, Gardner BP et al. Intrathecal baclofen for long-term treatment of spasticity: a multi-centre study. J Neurol Neurosurg Psychiatry 1989; 52:933-939.
Penn RD. Intrathecal baclofen for spasticity of spinal origin: seven years of experience. J Neurosurg 1992 ;77:236-240.
Possover M, Schurch B, Henle KP. New strategies of pelvic nerves stimulation for recovery of pelvic visceral functions and locomotion in paraplegics. Neurourol Urodyn 2010 Nov; 29(8).
Rayegani, S. M., Shojaee, H., Sedighipour, L., Soroush, M. R., Baghbani, M., & Amirani, O. B. (2011). The effect of electrical passive cycling on spasticity in war veterans with spinal cord injury. Frontiers in Neurology; 2(39):1-7.
Richardson D, Edwards S, Sheean GL, Greenwood RJ, Thompson AJ. The effect of botulinum toxin on hand function after incomplete spinal cord injury at the level of C5/6: a case report. Clin Rehabil 1997; 11(4):288-292.
Shields RK, Dudley-Javoroski S. Monitoring standing wheelchair use after spinal cord injury: a case report. Disabil Rehabil 2005; 27:142-146.
Simpson DM, Alexander DN, O’Brien CF, Tagliati M, Aswad AS, Leon JM et al. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology 1996; 46(5):1306-1310.
Simpson DM. Clinical trials of botulinum toxin in the treatment of spasticity. Muscle Nerve Suppl 1997; 6:S169-S175.
Smith SJ, Ellis E, White S, Moore AP. A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin Rehabil 2000; 14(1):5-13.
Snow BJ, Tsui JK, Bhatt MH, Varelas M, Hashimoto SA, Calne DB. Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol 1990; 28(4):512-515.
Thoumie P, Le CG, Beillot J, Dassonville J, Chevalier T, Perrouin-Verbe B et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia 1995; 33(11):654-659.
Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatry 1990; 53(9):754-763.
Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R et al. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev 2002; 39:53-61.
Wasiak J, Hoare B, Wallen M. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy. Cochrane Database Syst Rev 2004; (4):CD003469.
Lechner H, Kakebeeke T, Hegemann D, Baumberger M. The effect of hippotherapy on spasticity and on mental well-being of persons with spinal cord injury. Arch Phys Med Rehabil 2007;88:1241-8.
Nardone R, Holler Y, Thomschewski A, et al. rTMS modulates reciprocal inhibition in patients with traumatic spinal cord injury. Spinal Cord 2014;52:831-5.
Benito J, Kumru H, Murillo N, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spin Cord Injury Rehabil 2012;18:106-12.
Kumru H, Vidal J, Kofler M, Portell E, Valls-Sole J. Alterations in excitatory and inhibitory brainstem interneuronal circuits after severe spinal cord injury. Journal of Neurotrauma, 2010;27:721-8.
Image credits:
- Muscle strain ©Kylie Mhai, CC BY 3.0 US
- Modified from: Dorsiplantar ©Connexions, CC BY 3.0
- Image ©SCIRE
- Imgnotraçat arc reflex eng ©MartaAguayo, CC BY-SA 3.0
- Image ©SCIRE
- Image ©SCIRE
- Insomnia ©Gan Khoon Lay, CC BY 3.0 US
- Image ©SCIRE
- Standing frame ©Memasa, CC BY-SA 3.0
- Image ©SCIRE
- Intrathecal-pump-cartoon ©Anand Swaminathan, CC BY-SA 3.0
- Pills ©Nikita Kozin, CC BY 3.0 US
- Treatment ©Royal@design, CC BY 3.0 US
- Surgery ©Healthcare Symbols, CC0 1.0
- Neuro-ms ©Baburov, CC BY-SA 4.0
Disclaimer: This document does not provide medical advice. This information is provided for educational purposes only. Consult a qualified health professional for further information or specific medical advice. The SCIRE Project, its partners and collaborators disclaim any liability to any party for any loss or damage by errors or omissions in this publication.