Author: SCIRE Community Team | Reviewer: Shannon Sproule | Published: 18 October 2017 | Updated: ~
Pressure mapping is a clinical technique that is used to help prevent pressure sores after spinal cord injury (SCI). This page outlines how pressure mapping is used after SCI.
Key Points
- Pressure mapping is a technique that involves the use of a pressure-sensitive mat and computer system to measure the amount of pressure between a person’s body and a supporting surface.
- Pressure mapping is used to help identify areas of excess pressure that may contribute to pressure sores. This can be used to help make decisions about surface options (like wheelchair cushions and bedding) and find out how effective a person’s pressure relief techniques are.
- This technique involves the use of a flexible mat containing pressure sensors that is placed on the surface being tested and then the person is positioned on the mat. The mat is connected to a computer system that creates a color-coded diagram showing areas of pressure.
- Pressure mapping is considered to be a useful tool for making decisions about reducing pressure that is used as a decision-making and educational tool. There is a lack of research on whether pressure mapping directly helps to reduce pressure sores after SCI.
Pressure mapping is a technique that is used to identify areas of pressure between a person’s body and a supporting surface like a cushion or chair. A thin, pressure-sensitive mat and computer system are used to develop a map showing areas of pressure where the body contacts the surface. This technique is most often used to identify areas of high pressure associated with wheelchair seating that could contribute to the development of pressure sores.
See our article on Pressure Sores to learn more!
Pressure mapping is also used on other such as mattresses, toilet seats, car seats, sports equipment, or any other surface a person sits or lies on for periods of time that may influence their skin health.
People with spinal cord injuries have an increased risk of developing skin lesions called pressure sores. Pressure sores occur for many reasons, including increased pressure on vulnerable areas of the skin. Bony areas that come into contact with support surfaces, like the sit bones and tailbone in sitting, are highly vulnerable to increased pressure.
Learn how proper wheelchair seating can reduce the risk of pressure sores.
Pressure mapping is used to determine areas of increased pressure in certain postures on specific surfaces. It may be used to assess areas of pressure on various surfaces, such as chairs, beds, sofas, car seats, and toilet seats. This information can be used to develop strategies to reduce pressure and improve comfort in these situations.
Pressure mapping may be used as a tool to aid decision-making when selecting support surfaces and equipment, such as assessing which wheelchair cushions provide the best pressure relief for you. Pressure mapping is also used to assess the effectiveness of pressure relief techniques like weight-shifting by providing real-time feedback about the pressure during the performance of these techniques.

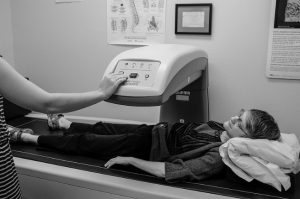
Pressure map placed on a wheelchair (left), flexible pressure map (center), and diagram of pressure of a person’s buttocks in sitting (right). Areas of pressure are indicated from high pressures in red (around the sit bones) to lower pressures in blue.3
A flexible mat containing pressure sensors is placed on the surface to be tested. The person being assessed then sits on the mat. Information about pressure between their body and the seat is picked up by sensors in the mat and sent to a computer, where it is translated into a color coded diagram.
The diagram displays the pressure recorded at each sensor in the mat by number and displays areas of high and low pressure with different colors. A clinician then determines the corresponding areas on the body through a hands-on physical examination. This technique is used together with other assessments of pressure sore risk to make recommendations for reducing areas of high pressure. There may be variation in the procedures used for pressure mapping in different settings.
Pressure mapping can be influenced by many aspects of how the procedure is done. For example, how the person positions themselves at the time of the reading and how long the person sits on the mat before the readings are taken can change the findings. There are also different types of systems that collect information about pressure differently. These factors and other concerns have led to disagreement among experts about how to best understand and interpret the results of pressure mapping for clinical use.
In addition, pressure is just one factor that contributes to pressure sores. Friction, moisture, age, body composition, time spent sitting, and many other factors also contribute to pressure sores. These factors cannot be detected by pressure mapping, which limits the use of pressure mapping as a stand-alone tool.
Pressure mapping also requires special equipment and trained health providers which may not be available in settings outside of major rehabilitation centers.
Pressure mapping is considered by experts to be a useful tool for understanding pressure and making decisions about pressure relief. At this time, most of the research that has been done on whether pressure mapping is effective for preventing pressure sores has been done in populations outside of SCI. We do not know if pressure mapping is effective for preventing of pressure sores in people with SCI.
Pressure mapping is clinical tool that may be used in rehabilitation centers to help assess the risk of pressure sores. It is considered to be a valuable tool for making decisions about reducing pressure as well as a useful educational tool for understanding pressure in different positions. There is a lack of research on whether pressure mapping directly helps to reduce pressure sores after SCI.
Hsieh J, McIntyre A, Wolfe D, Lala D, Titus L, Campbell K, Teasell R. (2014). Pressure Ulcers Following Spinal Cord Injury. In Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Noonan VK, Loh E, McIntyre A, editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0. 1-90.
Available from: https://scireproject.com/evidence/rehabilitation-evidence/skin-integrity-pressure-injuries/
Barnett RI, Shelton FE. Measurement of support surface efficacy: pressure. Adv Wound Care 1997; 10(7):21-9.
Brienza DM, Karg PE, Geyer MJ, Kelsey S, Trefler E. The relationship between pressure ulcer incidence and buttock-seat cushion interface pressure in at-risk elderly wheelchair users. Arch Phys Med Rehabil 2001; 82(4):529-33.
Eitzen I. Pressure mapping in seating: a frequency analysis approach. Arch Phys Med Rehabil 2004; 85(7):1136-40.
Ferguson-Pell M, Cardi MD. Prototype development and comparative evaluation of wheelchair pressure mapping system. Assist Technol 1993; 5(2):78-91.
Hamanami K, Tokuhiro A, Inoue H. Finding the optimal setting of inflated air pressure for a multi-cell air cushion for wheelchair patients with spinal cord injury. Acta Med Okayama 2004; 58(1):37-44.
Hanson D, Langemo D, Anderson J, Hunter S, Thompson P. Pressure mapping: seeing the invisible. Adv Skin Wound Care 2006; 19(8):432-4.
Henderson JL, Price SH, Brandstater ME, Mandac BR. Efficacy of three measures to relieve pressure in seated persons with spinal cord injury. Arch Phys Med 1994;75:535-9.
Jan YK, Brienza DM. Technology for Pressure Ulcer Prevention. Top Spinal Cord Inj Rehabil; 2006; 11(3):30-41.
Kernozek TW, Lewin JE. Seat interface pressures of individuals with paraplegia: influence of dynamic wheelchair locomotion compared with static seated measurements. Arch Phys Med Rehabil 1998; 79(3):313-6.
Rondorf-Klym LM, Langemo D. Relationship between body weight, body position, support surface, and tissue interface pressure at the sacrum. Decubitus 1993; 6(1):22-30.
Shelton F, Barnett R, Meyer E. Full-body interface pressure testing as a method for performance evaluation of clinical support surfaces. Appl Ergon 1998; 29(6):491-7.
Stinson MD, Porter-Armstrong A. Seating and pressure support needs of people with cancer in the cervix or rectum: a case series on the clinical usefulness of pressure mapping assessment. Euro J Cancer Care 2007; 17:298-305.
Stinson MD, Porter-Armstrong A, Eakin P. Seat-interface pressure: a pilot study of the relationship to gender, body mass index, and seating position. Arch Phys Med Rehabil 2003; 84(3):405-9.
Sonenblum SE, Sprigle SH. The impact of tilting on blood flow and localized tissue loading. J Tissue Variability 2011;20:3-13.
Taule T, Bergfjord EE, Lunde T, Stokke BH, Storlind H, Sorheim MV et al. Factors influencing optimal seating pressure after spinal cord injury. Spinal Cord 2013;51:273-7.
Image credits
- Veterans wheelchair games 2009 ©U.S. Air Force photo/Staff Sgt. Desiree N. Palacios, CC0 1.0
- Reprinted with permission of the copyright holder, Gordian Medical, Inc. dba American Medical Technologies (courtesy of National Pressure Ulcer Advisory Panel).
- Image ©Cho KH, Beom J, Yuk JH, Ahn SC, CC BY-NC 4.0
- Sit ©Rudez Studio, CC BY 3.0 US


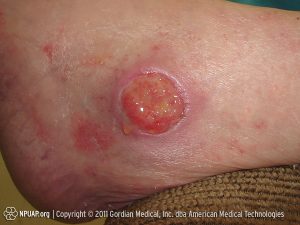
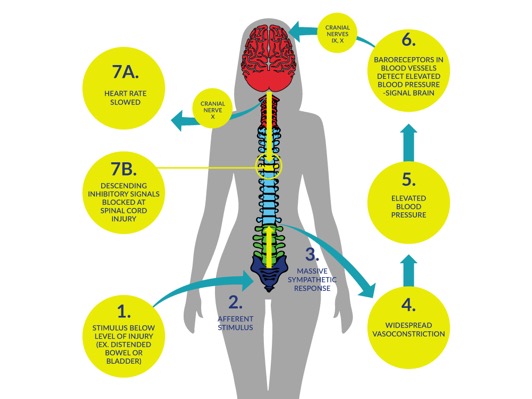

 Autonomic dysreflexia can be life threatening when severe. If left untreated, uncontrolled elevated blood pressure can lead to serious conditions like stroke, heart attack, detached retinas, seizures, and even death.
Autonomic dysreflexia can be life threatening when severe. If left untreated, uncontrolled elevated blood pressure can lead to serious conditions like stroke, heart attack, detached retinas, seizures, and even death.
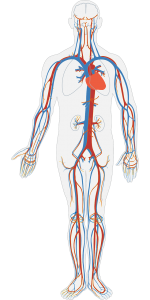 When the body below the SCI detects strong stimulation, it activates the sympathetic nervous system, causing the constriction of blood vessels in the lower body. This causes blood pressure to rise.
When the body below the SCI detects strong stimulation, it activates the sympathetic nervous system, causing the constriction of blood vessels in the lower body. This causes blood pressure to rise.
 If the steps above do not reduce blood pressure and it remains high (150 mmHg or above), seek emergency medical attention by calling an ambulance or visiting an emergency department. Emergency medical treatments involve the use of medications that rapidly lower blood pressure. The health providers may also run a series of tests to identify the cause of the episode if one has not been found.
If the steps above do not reduce blood pressure and it remains high (150 mmHg or above), seek emergency medical attention by calling an ambulance or visiting an emergency department. Emergency medical treatments involve the use of medications that rapidly lower blood pressure. The health providers may also run a series of tests to identify the cause of the episode if one has not been found. Bladder problems are the most common triggers of autonomic dysreflexia. Methods that may be used to prevent bladder problems from triggering autonomic dysreflexia include:
Bladder problems are the most common triggers of autonomic dysreflexia. Methods that may be used to prevent bladder problems from triggering autonomic dysreflexia include: Bowel problems are another common trigger of autonomic dysreflexia. Methods that may be used to prevent bowel problems from triggering autonomic dysreflexia include:
Bowel problems are another common trigger of autonomic dysreflexia. Methods that may be used to prevent bowel problems from triggering autonomic dysreflexia include: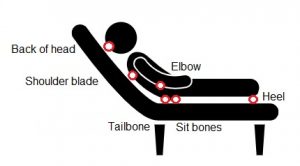 Pressure ulcers, ingrown toenails, and burns can all be additional causes of autonomic dysreflexia. Methods that may be used to prevent skin problems from triggering autonomic dysreflexia include:
Pressure ulcers, ingrown toenails, and burns can all be additional causes of autonomic dysreflexia. Methods that may be used to prevent skin problems from triggering autonomic dysreflexia include:




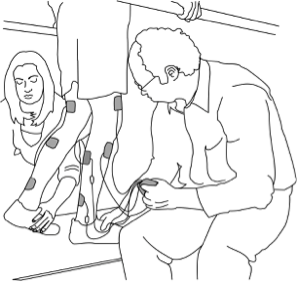
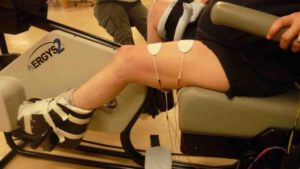
 There are some situations in which FES may be unsafe to use. This not a complete list, speak to a health provider about your health history and whether FES is safe for you.
There are some situations in which FES may be unsafe to use. This not a complete list, speak to a health provider about your health history and whether FES is safe for you. Studies have shown that both FES arm exercise and FES cycling helps to maintain or improve strength after SCI. However, FES cycling may be more effective for maintaining strength after injury than improving strength that has already been lost. This is supported by moderate evidence from five studies.
Studies have shown that both FES arm exercise and FES cycling helps to maintain or improve strength after SCI. However, FES cycling may be more effective for maintaining strength after injury than improving strength that has already been lost. This is supported by moderate evidence from five studies. Fifteen studies have looked at FES for improving many different aspects of fitness after SCI. Taken altogether, these studies provide weak evidence that FES training done at least 3 days per week for 2 months helps to improve many aspects of cardiovascular fitness after SCI.
Fifteen studies have looked at FES for improving many different aspects of fitness after SCI. Taken altogether, these studies provide weak evidence that FES training done at least 3 days per week for 2 months helps to improve many aspects of cardiovascular fitness after SCI. Studies show that FES improves walking speed and distance in people with both incomplete and complete SCI. Some of these studies also showed that regular use of FES carried over to improve walking even without FES. This is supported by weak evidence from eight studies.
Studies show that FES improves walking speed and distance in people with both incomplete and complete SCI. Some of these studies also showed that regular use of FES carried over to improve walking even without FES. This is supported by weak evidence from eight studies. Although it is commonly thought that increased muscle bulk from FES will reduce the risk of pressure sores, there are not very many studies which have looked at whether this actually happens. One study provides weak evidence that FES cycling for 2 years reduced the number of pressure ulcers that occurred after SCI. Another study showed that regular FES cycling showed a trend toward reducing seat pressures.
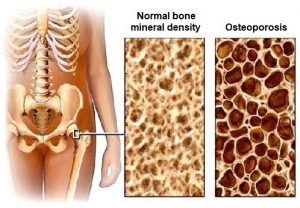
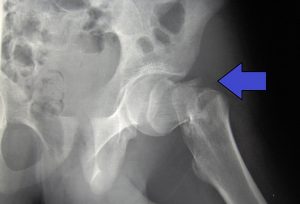
Although it is commonly thought that increased muscle bulk from FES will reduce the risk of pressure sores, there are not very many studies which have looked at whether this actually happens. One study provides weak evidence that FES cycling for 2 years reduced the number of pressure ulcers that occurred after SCI. Another study showed that regular FES cycling showed a trend toward reducing seat pressures. Research studies show that FES cycling does not prevent bone loss after SCI (moderate evidence from two studies). However, it may help to increase bone density that has already been lost, although the evidence for this is conflicting (based on six studies). It is not clear whether any gains in bone density last long-term or if continued FES treatment is needed for them to be maintained.
Research studies show that FES cycling does not prevent bone loss after SCI (moderate evidence from two studies). However, it may help to increase bone density that has already been lost, although the evidence for this is conflicting (based on six studies). It is not clear whether any gains in bone density last long-term or if continued FES treatment is needed for them to be maintained.

 Depression is diagnosed through interviews with a health provider such as a doctor or psychologist. The health provider will ask questions about mood and a number of other symptoms, and may have you complete questionnaires about your symptoms.
Depression is diagnosed through interviews with a health provider such as a doctor or psychologist. The health provider will ask questions about mood and a number of other symptoms, and may have you complete questionnaires about your symptoms.
 Antidepressant medications (antidepressants) are another common treatment option for depression. There is a wide range of different antidepressants that may be used. Some antidepressant medications can treat sleep, nerve pain and mood simultaneously, and these are often used in people with SCI. Antidepressants are prescribed by medical doctors.
Antidepressant medications (antidepressants) are another common treatment option for depression. There is a wide range of different antidepressants that may be used. Some antidepressant medications can treat sleep, nerve pain and mood simultaneously, and these are often used in people with SCI. Antidepressants are prescribed by medical doctors. Exercise is now becoming more widely known as a treatment option for depression. Exercise may help treat depression because it helps to reduce pain and stress, causes the release of “feel-good” chemicals like endorphins, and helps to maintain mobility and quality of life.
Exercise is now becoming more widely known as a treatment option for depression. Exercise may help treat depression because it helps to reduce pain and stress, causes the release of “feel-good” chemicals like endorphins, and helps to maintain mobility and quality of life.











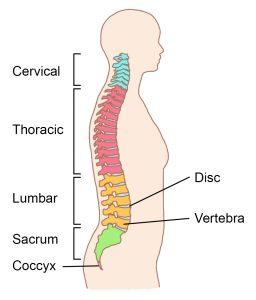

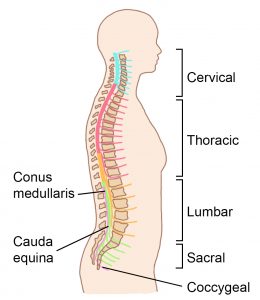
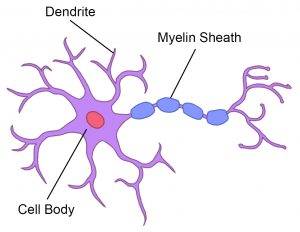
 The spinal cord provides a pathway for movement commands to travel from the brain to the muscles. This is called motor function. Neurons that send movement commands are called motor neurons.
The spinal cord provides a pathway for movement commands to travel from the brain to the muscles. This is called motor function. Neurons that send movement commands are called motor neurons.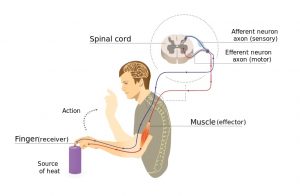



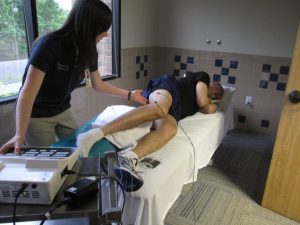







 Doing research in populations with SCI is essential to improving treatment, rehabilitation, and management options for people with SCI, but there are some obstacles. Some research limitations unique to research in SCI populations include:
Doing research in populations with SCI is essential to improving treatment, rehabilitation, and management options for people with SCI, but there are some obstacles. Some research limitations unique to research in SCI populations include:


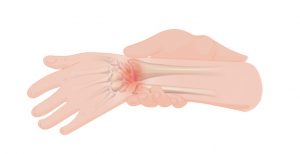
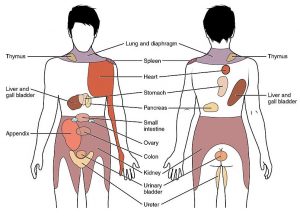
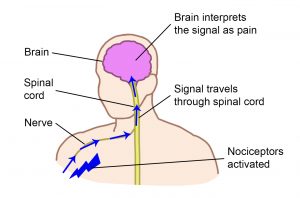
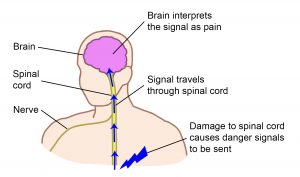
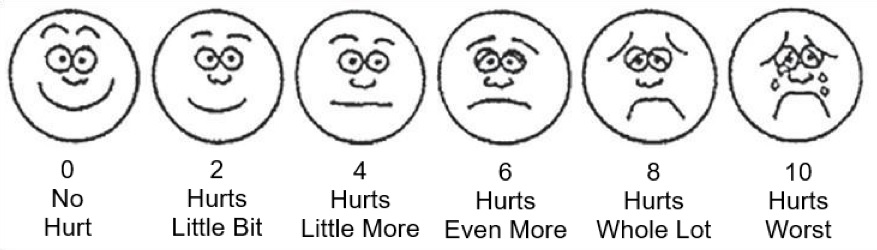




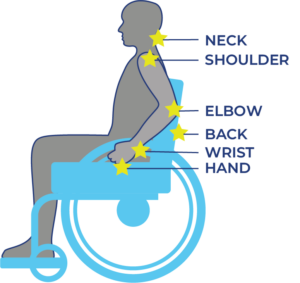 Musculoskeletal pain is caused by problems in the muscles, joints or bones. It is a common problem for all people as they get older, including those with SCI. Most people aging with SCI experience increased musculoskeletal pain in the upper extremities (shoulder and arm). Other common pain spots include the elbows, wrists, and hands. Issues with posture and seating can cause neck and back pain.
Musculoskeletal pain is caused by problems in the muscles, joints or bones. It is a common problem for all people as they get older, including those with SCI. Most people aging with SCI experience increased musculoskeletal pain in the upper extremities (shoulder and arm). Other common pain spots include the elbows, wrists, and hands. Issues with posture and seating can cause neck and back pain.